News
Andy Murray undergoes hip resurfacing surgery in London 
Andy Murray has undergone hip surgery in one final attempt to save his elite tennis career. Published in The Telegraph.
Last modified: Tue, 05 Feb 2019 14:29:23 GMT
J&J Pays $120 Million to Resolve State Suits Over Hip Marketing
Article published in Bloomberg.
Last modified: Tue, 05 Feb 2019 14:21:16 GMT
Supreme Court closes case against Johnson and Johnson
Centre informs Bench that compensation will be given to victims of faulty hip implants. Article published in The Hindu.
Last modified: Tue, 05 Feb 2019 13:56:40 GMT
J&J Starts Settling Suits Over Pinnacle Hip Defect Claims
Article published in Bloomberg.
Last modified: Tue, 05 Feb 2019 13:26:19 GMT
Firm pays out to NHS over defective hip replacements
DePuy recalled metal-on-metal system in 2010 and has covered cost of corrective surgeries. Article published by The Guardian.
Last modified: Wed, 30 Jan 2019 15:02:31 GMT
Faulty medical implants harm patients around world
Twitter post by the Guardian.
Last modified: Wed, 30 Jan 2019 14:55:02 GMT
The Implant Files
A special investigation by The Irish Times in association with the International Consortium of Investigative Journalists.
Last modified: Wed, 30 Jan 2019 14:50:30 GMT
Suspect device: the all-metal hip replacement that caused untold hardship
In excess of 1,000 Irish patients have issued proceedings against DePuy. Article published in the Irish Times.
Last modified: Wed, 30 Jan 2019 14:47:04 GMT
SC seeks Centre’s response to PIL on J&J 'faulty' hip implant
Controversy on Johnson & Johnson's faulty hip implants reached the Supreme Court.
Last modified: Wed, 23 Jan 2019 09:47:08 GMT
J&J to work with India on compensation for recalled hip implants
Johnson & Johnson (J&J) said it would work with the Indian government to compensate patients who had suffered from hip implants that were recalled by the U.S. healthcare firm eight years ago after data showed high failure rates.
Last modified: Tue, 22 Jan 2019 12:40:26 GMT
Concern over joint replacement failures due to lack of orthopaedic register
'Ireland is heavily reliant on UK guidance in the absence of a national register here' says the Irish Times.
Last modified: Tue, 28 Aug 2018 12:55:23 BST
The Depuy Pinnacle Metal on Metal Hip Litigation
Colin Gee and others -v- Depuy International Limited (The Depuy Pinnacle Metal on Metal Hip Litigation)
Last modified: Tue, 21 Aug 2018 13:58:46 BST
Hundreds of patients lose battle over allegedly 'defective' hip implants 
Article published in The Telegraph.
Last modified: Tue, 21 Aug 2018 13:53:21 BST
‘My hip replacement left me in constant pain'
BBC News article on the recent court ruling that manufacturer DePuy was not liable.
Last modified: Tue, 21 Aug 2018 13:50:33 BST
Patients lose hip replacement court case
BBC News: Hundreds of patients have lost the first round of a legal battle for compensation at the High Court over allegedly "defective" hip implants.
Last modified: Tue, 21 Aug 2018 13:46:29 BST
Canada has a 'big blind spot' on medical device safety: study
CTV News article. Unlike medications, which undergo rigid pre-market testing, medical devices are often approved with little or no clinical testing in Canada.
Last modified: Tue, 21 Aug 2018 11:18:55 BST
Johnson & Johnson wins appeal to overturn $151 million hip implant verdict
Reuters 26/4/2018: Johnson & Johnson secured a favorable U.S. appellate court ruling, overturning a verdict that had awarded $151 million to five people who blamed injuries they suffered on the company’s Pinnacle hip implant devices.
Last modified: Tue, 21 Aug 2018 11:09:25 BST
Future growth for JRI following it’s acquisition by AK Medical, China
Award-winning healthcare company JRI Orthopaedics has been acquired by China’s leading orthopaedic implant company AK Medical in a £16.7M deal.
Last modified: Tue, 21 Aug 2018 09:53:50 BST
Woman (81) with DePuy hip replacement awarded €321k by High Court
Article published in Independent.ie. An 81-year old woman who sued over her DePuy hip replacement has been awarded €321,000 by the High Court.
Last modified: Tue, 27 Feb 2018 13:08:08 GMT
Engineering solutions for the future of modern medicine
Working alongside clinicians, biomedical engineers are on the frontline of major advances in healthcare. Published in the Guardian.
Last modified: Tue, 27 Feb 2018 12:43:50 GMT
We're living longer but in poorer health, warns thinktank
Life expectancy is increasing, but the number of years of healthy life in retirement is not keeping up, with dramatic variations seen across the country. Article published in the Guardian.
Last modified: Tue, 27 Feb 2018 12:28:24 GMT
Johnson & Johnson hit with $247 million verdict in hip implant trial
A federal jury in Dallas ordered Johnson & Johnson and its DePuy Orthopaedics unit to pay $247 million to six patients who said they were injured by defective Pinnacle hip implants. Article published by Reuters.
Last modified: Tue, 27 Feb 2018 12:16:14 GMT
BBC News: Hip implant patients sue manufacturer
Hundreds of people who received allegedly faulty hip replacements are suing the manufacturer at the High Court.
Last modified: Thu, 19 Oct 2017 10:06:27 BST
Metal-on-metal hip implant patients sue manufacturer in 'biggest ever' class act
Telegraph 14th October 2017. Hundreds of patients claiming compensation for “failed” metal-on-metal hip implants will take a manufacturer to court tomorrow in one of the largest class actions of its kind.
Last modified: Thu, 19 Oct 2017 09:55:52 BST
$89 Million Rids Wright Medical of Metal-on-Metal Hip Lawsuits
Wright Medical Technology, Inc. (WMT) and plaintiffs in the Conserve metal-on-metal hip litigation have agreed on a "comprehensive" settlement to settle almost all the last claims against the company.
Last modified: Mon, 16 Oct 2017 14:46:18 BST
National Joint Registry 2017
NJR's 14th Annual Report is now published with information, data and analyses on hip, knee, ankle, elbow and shoulder joint replacement surgery.
Last modified: Thu, 12 Oct 2017 15:25:39 BST
India introduces price controls for knee implants
Reuters report that India has capped prices of orthopaedic knee implants, in the country’s latest move to bring down prices of medical devices.
Last modified: Thu, 12 Oct 2017 13:47:46 BST
2016 Report to the Public About Hip and Knee Replacements
AJRR's first-ever patient annual report
Last modified: Thu, 12 Oct 2017 13:32:35 BST
When your body becomes eligible for an upgrade
BBC news article on 'What will happen when humans decide to become robots?'
Last modified: Thu, 12 Oct 2017 13:16:01 BST
Fresh concerns for ‘metal-on-metal’ hip implant patients
HSE safety alert sent to all State hospitals says patients need to undergo regular testing (The Irish Times)
Last modified: Thu, 12 Oct 2017 11:32:50 BST
Near doubling in failure rates for metal-on-metal hips behind alert
Telegraph article. Checks on all patients with metal-on-metal hips were prompted by a near-doubling in failure rates for the most common of the devices, records reveal.
Last modified: Tue, 04 Jul 2017 12:57:32 BST
'Metal-on-metal' hip implant patients recalled for tests over toxicity fears
Article published in the Guardian. Alert issued by regulatory agency calls for for MRI scans and blood tests on expanded pool from 56,000 patients using devices believed to be at risk of bone or muscle damage.
Last modified: Tue, 04 Jul 2017 10:19:34 BST
More than 50,000 Britons with MoM hips told to have X-rays and blood tests
Article in the Telegraph. More than 50,000 patients with “metal-on-metal” hips are being told to undergo X-rays and blood tests after watchdogs found they are far more toxic than was thought.
Last modified: Tue, 04 Jul 2017 10:13:18 BST
Hip firm 'cashed in' on unsafe implants which left thousands of patients in pain
A director at the company behind hip implants which left thousands of British patients in pain boasted that its sales figures were "on fire" as a result of operations to replace unsafe parts.
Last modified: Thu, 27 Apr 2017 13:28:49 BST
Hip implant maker was alerted to safety fears
Article published in the Telegraph. Hip implant maker was alerted to safety fears, as surgeon told them: ‘It borders on unethical to market’
Last modified: Thu, 27 Apr 2017 13:30:41 BST
The people with arthritis struggling to work
How do people juggle staying in work with a painful and debilitating condition like arthritis
Last modified: Wed, 08 Mar 2017 13:17:44 GMT
Pain-level rationing of hip and knee surgery due to cash crisis, admits NHS
Article in The Guardian. Official says financial pressure to blame for West Midlands plan to offer transplants only to those who cannot sleep or do daily tasks.
Last modified: Wed, 08 Mar 2017 11:09:50 GMT
Update: Judge halves $1 billion award in J&J hip implants case
Reuters: A U.S. judge almost halved the award in a December jury verdict that ordered Johnson & Johnson and its DePuy Orthopaedics unit to pay more than $1 billion to plaintiffs in six lawsuits.
Last modified: Tue, 24 Jan 2017 14:43:22 GMT
Woman awarded €700,000 damages over hip implant
Article in the Irish Times. High Court award is first made by a judge in a case over DePuy products.
Last modified: Tue, 24 Jan 2017 13:40:00 GMT
J&J Ordered to Pay Record $1 Billion for Faulty Hip Implants 
Article in Bloomberg states that J&J faces almost 9,000 lawsuits over its metal-on-metal hips
Last modified: Tue, 24 Jan 2017 13:34:21 GMT
National Joint Registry defends THR as procedure that improves patient function 
Article published in Orthopaedics Today Europe, November 2016.
Last modified: Tue, 24 Jan 2017 13:15:10 GMT
Patients must understand options, says Royal College of Surgeons
Article published in the Guardian.
Last modified: Tue, 24 Jan 2017 13:03:25 GMT
Wright Medical Completes Divestiture of Large Joints Business
Article published in Orthopaedic Design and Technology
Last modified: Tue, 24 Jan 2017 12:58:07 GMT
2016 AOA National Joint Replacement Registry
The 2016 Australian Orthopaedic Association National Joint Replacement Registry is now published.
Last modified: Tue, 24 Jan 2017 12:51:00 GMT
NJR 2016 now published
The NJR's 13th Annual Report has been published with data and analyses on hip, knee, ankle, elbow and shoulder joint replacement surgery.
Last modified: Tue, 24 Jan 2017 12:40:26 GMT
New data demonstrates benefits to patients from a range of elective surgeries
Annual data for Patient Reported Outcome Measures shows patients who have undergone elective inpatient surgery for four common elective procedures including hip and knee replacement, are reporting significant health gains.
Last modified: Wed, 14 Sep 2016 12:08:22 BST
Zimmer Biomet strikes multiyear orthopedics deal in India
Zimmer Biomet struck a multiyear deal with India healthcare leaders to bring better orthopedic care to the country.
Last modified: Thu, 11 Aug 2016 09:24:24 BST
Future of an ageing population
A UK government report looking at the challenges and opportunities of an ageing society.
Last modified: Thu, 11 Aug 2016 09:14:41 BST
One in three hip implants were the wrong size, study finds 
Report by The Telegraph. A “significant” number of hip replacements surgically implanted into British patients were the wrong size, a scientific study has found.
Last modified: Wed, 25 May 2016 14:23:49 BST
J&J slapped with $500M verdict in metal-on-metal hip implant cases
Article published in FierceMedicalDevices
Last modified: Thu, 21 Apr 2016 09:05:05 BST
Johnson & Johnson hit with $500 million verdict in hip implant trial
Article published by Reuters.
Last modified: Thu, 21 Apr 2016 08:57:13 BST
J&J Ordered to Pay $502 Million Over Pinnacle Hip Failures
Article published in Bloomberg.
Last modified: Thu, 21 Apr 2016 08:54:20 BST
BBC News: Rise in hip replacements for under 60s
The number of hip replacement operations on people aged under 60 has risen 76% in the last decade, NHS figures for England reveal.
Last modified: Wed, 16 Mar 2016 13:02:43 GMT
I used to swim and run, then a metal hip implant crucified me
Colin Gee, 72, says operation left in him constant pain as DePuy faces thousands of court claims.
Last modified: Thu, 04 Feb 2016 14:22:31 GMT
Patients given hip implants made the wrong size
Investigation: Manufacturer DePuy admits “an error in the measuring techniques” when making metal-on-metal implants.
Last modified: Thu, 04 Feb 2016 14:17:13 GMT
Process speeds up faulty hip implant claims
From the Irish Examiner concerning ASR metal-on-metal hips.
Last modified: Thu, 04 Feb 2016 14:23:58 GMT
"Pope McGlamry: $11 Million Verdict in First Wright Conserve Hip Bellwether Tria
The jury in a trial against Wright Medical Technology, Inc. for product liability and misrepresentation claims relating to their Conserve metal-on-metal hip implant device, awarded $11 million in favour of the Plaintiff.
Last modified: Thu, 26 Nov 2015 13:44:00 GMT
Judge orders ‘innovative’ solutions in DePuy hip implant cases
DePuy International to bring motion before High Court offering alternative dispute resolution. Reported in the Irish Times.
Last modified: Thu, 26 Nov 2015 13:14:12 GMT
BBC News - Britain's oldest person Gladys Hooper has hip op at 112 
A 112-year-old woman from the Isle of Wight is believed to be the oldest person in the world to have had a hip replacement.
Last modified: Thu, 26 Nov 2015 13:05:41 GMT
Hip implant maker claims surgical funder inflated patients' bills
A unit of Johnson & Johnson that makes artificial hips has accused a surgical funding company of seeking excessive profits from financing surgery for patients suing over the devices.
Last modified: Thu, 26 Nov 2015 11:03:12 GMT
2015 National Joint Registry now published 
The NJR 12th Annual Report is now available to download.
Last modified: Thu, 26 Nov 2015 10:44:31 GMT
Metal-on-metal hip implants
From 2011, the Dutch Health Care Inspectorate report on metal-on-metal hips
Last modified: Thu, 26 Nov 2015 10:46:25 GMT
Half of decade-old metal hip implants defective
Article in the New Zealand Herald. Half of the metal-on-metal hip implants received by thousands of New Zealanders about a decade ago experienced significant defects, a Herald investigation has found.
Last modified: Thu, 26 Nov 2015 10:48:05 GMT
The F.D.A.’s Medical Device Problem
Incredibly, legislation that the House of Representatives passed in July 2015 would severely weaken, not strengthen, the F.D.A.’s already ineffective regulatory scheme for medical devices. Article published in the New York Times.
Last modified: Wed, 05 Aug 2015 13:00:52 BST
PROMs clinical case study: data informs clinical practice
Consultant Trauma and Orthopaedic Surgeon Mike Reed has used Patient Reported Outcomes Measures (PROMs) data to inform medical practice across the Northumbria NHS Healthcare Foundation trust.
Last modified: Wed, 05 Aug 2015 12:38:06 BST
Crown Dependency Political News
Isle of Man joins the National Joint Registry
Last modified: Thu, 16 Jul 2015 10:10:01 BST
Zimmer Completes Biomet Acquisition, Solidifies Leadership in Knee and Hip Space
Zimmer completed its acquisition of Biomet, becoming the world’s second-largest orthopaedic device company and a clear leader in joint replacement.
Last modified: Tue, 07 Jul 2015 12:25:43 BST
Zimmer-Biomet Merger is a Done Deal 
Orthopedic Design and Technology. One of the largest deals consolidating two orthopaedic powerhouses finally has come to a close.
Last modified: Thu, 02 Jul 2015 15:02:27 BST
Birmingham Hip Resurfacing system. Update - recall and new hazard alert
From TGA Australia. Consumers and health professionals are advised that Smith & Nephew, in consultation with the TGA, is recalling certain unused Birmingham Hip Resurfacing (BHR) system components and issuing a related hazard alert update.
Last modified: Thu, 02 Jul 2015 14:43:27 BST
Smith & Nephew to Close U.K. Facility Next Year
Article published in Orthopaedic Design and Technology.
Last modified: Thu, 02 Jul 2015 14:39:14 BST
Plaintiffs win $4.5 million in trial over Wright hip device
Jurors in California state court awarded $4.5 million to plaintiffs in the first of several dozen lawsuits over Wright Medical Technology's Profemur R hip device to go to trial.
Last modified: Thu, 02 Jul 2015 14:29:55 BST
Statement regarding BHR System (FierceMedicalDevices)
Voluntary actions initiated to remove smaller size components and change Instructions for Use (IFU) following analysis of recent performance data.
Last modified: Thu, 02 Jul 2015 14:21:02 BST
Engineering for an ageing population
News archive posted by the Royal Academy of Engineering discussing the 21st Century Engineering for an Ageing Population Policy Statement from the IMechE.
Last modified: Thu, 02 Jul 2015 13:47:40 BST
Thousands of Patients Under Observation in Faulty Implant Scandal
Supplier knowingly sold defective implants that were distributed to health centres in Spain (article in Orthopedic Design and Technology).
Last modified: Thu, 28 May 2015 11:38:29 BST
Data registry: younger orthopedics patients are no healthier than older ones
FierceMedicalDevices reports that the Force-TJR data registry is following patients for life, 30,000 from 150 hospitals, thanks to a four-year, $12 million grant from the federal Agency for Healthcare Research & Quality.
Last modified: Tue, 12 May 2015 14:35:34 BST
Update on Birmingham Hip Modular Head- Urgent Field Safety Notice
Based on recent clinical and registry data, Smith & Nephew have issued a new urgent field safety notice related to the Birmingham Hip Modular Head. (Arthroplasty Watch.)
Last modified: Tue, 07 Apr 2015 14:08:32 BST
Patients with metal hips implants hit by illness
From Australia; A Sunday Morning Herald article.
Last modified: Tue, 07 Apr 2015 13:49:34 BST
DeltaLox acetabular shells used in hip implants
Australian Government, Department of Health, Therapeutic Goods Administration (TGA) hazard alert - higher than expected revision rate
Last modified: Tue, 07 Apr 2015 13:28:11 BST
British Orthopaedic Association: Getting It Right First Time (GIRFT)
A national review of adult elective orthopaedic services in England
Last modified: Tue, 07 Apr 2015 13:18:17 BST
Pseudotumor formation and serum ions after large head metal-on-metal stemmed THR
Paper published in Orthopaedic and Trauma Surgery: 'Pseudotumor formation and serum ions after large head metal-on-metal stemmed total hip replacement. Risk factors, time course and revisions in 706 hips'
Last modified: Tue, 07 Apr 2015 11:42:19 BST
Genesis II Knee System - non-porous tibial baseplate (used in knee replacements)
Australian Government, Department of Health, Therapeutic Goods Administration (TGA). Hazard alert - potential for implant failure due to poor fixation
Last modified: Tue, 07 Apr 2015 10:53:58 BST
Bloomberg "J&J to Pay As Much As $420 Million More in ASR Hip Accord"
Johnson & Johnson agreed to pay as much as $420 million more to resolve lawsuits over recalled hip implants that were excluded in 2013 from a $2.5 billion settlement of claims
Last modified: Thu, 05 Mar 2015 13:50:55 GMT
Hazard alert from TGA Australia: "Biomet M2a metal-on-metal total hip implants"
Hazard alert - higher than expected revision rate
Last modified: Thu, 05 Mar 2015 13:32:20 GMT
Knee Replacement Device Unapproved, but Used in Surgery
A firm sold 18,000 knee-replacement tools before the government called a halt.
Last modified: Wed, 04 Mar 2015 15:08:31 GMT
Johnson & Johnson Will Make Clinical Data Available to Outside Researchers
New York Times: The health care giant Johnson & Johnson has agreed to make detailed clinical trial data on its medical devices and diagnostic tests available to outside researchers through a collaboration with Yale University.
Last modified: Wed, 04 Mar 2015 11:41:56 GMT
From Irish Times "Court ruling may halt dozens of hip implant cases"
Dozens of cases being taken against the manufacturers of allegedly defective hip replacements may be struck out following a ruling by the High Court.
Last modified: Wed, 04 Mar 2015 10:56:48 GMT
Affinis Fracture ceramic head (used in shoulder replacements)
From TGA Australia "Affinis Fracture ceramic head (used in some shoulder replacements)" hazard alert
Last modified: Tue, 03 Mar 2015 14:38:52 GMT
FDA outlines UDI implementation plans for 2015
The FDA outlined its 2015 plans for Unique Device Identification. Article by Fierce Medical Devices.
Last modified: Tue, 03 Mar 2015 14:20:08 GMT
FDA delays UDI labelling requirements
FDA delays UDI labelling requirements by two years for some orthopaedic devices (article by Fierce Medical Devices)
Last modified: Tue, 03 Mar 2015 14:15:45 GMT
Think Twice Before Choosing Knee Replacement
From the New York Times Magazine discussing the want versus the need for knee replacement surgery
Last modified: Tue, 03 Mar 2015 14:09:45 GMT
Wright Medical Group and Tornier Agree to Merge
Fierce Medical Devices report that Wright Medical Group, Inc. and Tornier N.V. agree to merge creating a premier high-growth extremities-biologics company
Last modified: Tue, 11 Nov 2014 14:58:11 GMT
Johnson & Johnson Wins First Pinnacle Hip Implant Trial
An article published in Bloomberg reporting that a Johnson & Johnson (JNJ) unit’s design of a metal-on-metal version of its Pinnacle hip implants isn’t defective
Last modified: Thu, 06 Nov 2014 15:10:30 GMT
The 2014 Australian Joint Registry
The 2014 Australian Joint Registry, and the latest revision rates for joint implants, is now available
Last modified: Thu, 06 Nov 2014 14:54:38 GMT
The Placebo Effect Doesn’t Apply Just to Pills
Article from the New York Times
Last modified: Thu, 06 Nov 2014 14:47:20 GMT
JAMA Internal Medicine article
Lack of Publicly Available Scientific Evidence on the Safety and Effectiveness of Implanted Medical Devices
Last modified: Thu, 06 Nov 2014 14:27:19 GMT
National Joint Registry of England, Wales and Northern Ireland 2014
The latest National Joint Registry of England, Wales and Northern Ireland has now been released and is available on-line.
Last modified: Tue, 30 Sep 2014 14:34:52 BST
From Reuters "Smith & Nephew to pay $11 million in whistleblower suit" 
London-based medical device maker Smith & Nephew PLC has agreed to pay $11.3 million (6.9 million pounds) to settle allegations that it sold the U.S. government devices it claimed were U.S.-made but actually came from Malaysia
Last modified: Tue, 30 Sep 2014 14:36:31 BST
J&J's Pinnacle Hips Face First Trial on Poisoned Patients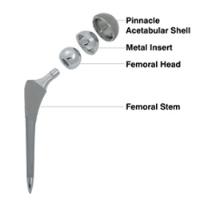
Bloomberg reports that Johnson & Johnson, which set aside $2.5 billion last year to resolve claims that 8,000 of its artificial hips were defective, faces new lawsuits over another line of hip implants blamed for poisoning patients.
Last modified: Tue, 30 Sep 2014 13:40:27 BST
Provisional Monthly PROMs in England – April 2012 to Mar 2013, May 2014 release
Patient Reported Outcome Measures (PROMs) in England show positive results from hip and knee replacement
Last modified: Wed, 06 Aug 2014 13:12:13 BST
Hazard alert - NexGen Complete Knee Solution MIS stemmed tibial component
From TGA Australia device alert "NexGen Complete Knee Solution MIS stemmed tibial component (various sizes)"
Last modified: Wed, 06 Aug 2014 12:57:24 BST
J&J, Oregon Settle in First State Deal Over Hip Implants
Bloomberg article: Johnson & Johnson to pay $4 million to resolve deceptive marketing claims over recalled metal-on-metal hips
Last modified: Thu, 17 Jul 2014 12:05:51 BST
MHRA "New Legislation on Medical Devices" 
All of the current legislation regulating medical devices is in the process of being revised at European level. This will replace the existing three European directives with two European regulations.
Last modified: Thu, 17 Jul 2014 11:47:47 BST
Stryker to buy Small Bone Innovations 
Stryker announces definitive agreement to acquire assets of Small Bone Innovations, Inc
Last modified: Thu, 17 Jul 2014 11:49:37 BST
Medical device adverse event reports - statistics for 2013
This report from the Therapeutic Goods Administration's Office of Product Review (OPR) includes an overview of several aspects of post-market monitoring of medical devices in Australia
Last modified: Wed, 18 Jun 2014 13:23:30 BST
India issues recall, commences criminal investigation into defective ASR hip
From ABC Australia: Health authorities in India have initiated a criminal investigation into how an international medical devices company failed to adequately follow up a worldwide recall notice on a defective hip implant device
Last modified: Wed, 28 May 2014 11:52:01 BST
Relation between surgeon volume and risk of complications after THR
BMJ article: Relation between surgeon volume and risk of complications after total hip arthroplasty: propensity score matched cohort study
Last modified: Wed, 28 May 2014 11:10:21 BST
MPs and peers call for greater patient power
Article in BMJ.
Last modified: Wed, 28 May 2014 10:52:06 BST
Orthopedics Today: Zimmer to combine with Biomet
Zimmer to purchase Biomet. The combined company will continue to be headquartered in Warsaw, Ind., US.
Last modified: Wed, 28 May 2014 10:26:19 BST
MEPs back stronger controls on medical devices
The European parliament has overwhelmingly approved stronger controls on medical devices; as reported in th BMJ.
Last modified: Wed, 28 May 2014 10:28:02 BST
Euro Parliament Sets Regs Changes inTrain April 3, 2014
The European Parliament is edging towards completion of the long-awaited steps that will spell a new regime for regulating medical devices in Europe
Last modified: Mon, 07 Apr 2014 09:34:55 BST
Stryker settles 8 more metal hip lawsuits
Injury Lawyer News reported eight plaintiffs suing over the company's Rejuvenate all-metal hip implants have reached a settlement.
Last modified: Mon, 07 Apr 2014 09:36:47 BST
Practical considerations for volumetric wear analysis of explanted hip joints
This Bone Joint Research paper discusses the use of coordinate measuring machines (CMMs) to provide estimates of total volumetric material loss of explanted prostheses to help understand device failure
Last modified: Mon, 24 Mar 2014 09:42:14 GMT
Getting it right first time
Improving the Quality of Orthopaedic Care within the National Health Service in England
Last modified: Tue, 11 Mar 2014 14:27:26 GMT
Cancer incidence and cause-specific mortality in MOM hip patients in Finland
This article assesses the risk of death and provides updated data on the risk of cancer related to metal-on-metal hip replacements in a population-based study with a mean follow-up of 4.6 (1–11) years.
Last modified: Tue, 11 Mar 2014 14:06:59 GMT
NICE guidance: Exercise key to managing osteoarthritis
Exercise is a core treatment in managing osteoarthritis, according to updated NICE guidance on the condition.
Last modified: Tue, 11 Mar 2014 13:56:00 GMT
Biomet reaches $56 million settlement over faulty hip replacements
Reuters article. U.S. medical device maker Biomet Inc will pay at least $56 million to settle a multi-district lawsuit relating to defective metal hip replacements, a court filing showed.
Last modified: Tue, 11 Mar 2014 13:02:41 GMT
Research requests for the National Joint Registry (NJR)
Considering a research request for NJR? 2014 submission process detailed here.
Last modified: Tue, 11 Mar 2014 12:03:08 GMT
MRHA Field Safety Notice for small (<48mm) ADEPT MoM hip resurfacings
MatOrtho has added a warning to the IFU for the ADEPT Hip Resurfacing System.
Last modified: Tue, 04 Feb 2014 14:05:48 GMT
Risk Stratification Algorithm for Management of Patients with Metal-on-Metal Hip
From Earl's View, this is a Concensus Statement of the American Association of Hip and Knee Surgeons, the Amerian Academy of Orthopaedic Surgeons and the Hip Society.
Last modified: Thu, 30 Jan 2014 15:10:27 GMT
Primary hip replacement prostheses and their evidence base: review of literature
This article determines the extent to which prostheses with no readily available evidence to support their use are being implanted in primary total hip arthroplasty.
Last modified: Thu, 30 Jan 2014 14:56:18 GMT
Stryker agrees to settle four metal hip lawsuits
Stryker agrees to settle four metal hip lawsuits as hundereds more loom.
Last modified: Thu, 30 Jan 2014 12:58:26 GMT
Mortality rates at 10 years after MoM hip resurfacing compared with total hips
Mortality rates at 10 years after metal-on-metal hip resurfacing compared with total hip replacement in England: retrospective cohort analysis of hospital episode statistics.
Last modified: Thu, 30 Jan 2014 11:34:59 GMT
DePuy Orthopaedics to pay $2.5 billion in ASR Hip System settlement
DePuy Orthopaedics announces settlement re ASR
Last modified: Mon, 25 Nov 2013 13:36:38 GMT
Tribology and Total Hip Arthroplasty Implants
Questions and answer session with Karl Knahr, MD on tribology and how it relates to total hip arthroplasty.
Last modified: Thu, 30 Jan 2014 11:15:30 GMT
Resurfacing Prostheses Found to Have Higher than Anticipated Revision Rate
Based on the 2013 National Joint Replacement Registry annual report (Australian Orthopaedic Association) some Cormet prostheses have been reported to have a significantly higher rate of revision.
Last modified: Thu, 30 Jan 2014 11:06:30 GMT
NHS hospitals to be banned from fitting MOM hip replacements after high failure-200x39.JPG)
Telegraph UK: NHS hospitals are to be banned from fitting most metal-on-metal hip replacements after a study found unacceptably high failure rates among implants in 17,000 patients.
Last modified: Thu, 30 Jan 2014 10:46:21 GMT
High Frequency of Adverse Local Tissue Reactions (ALTR) in Patients With MOM THA
High Frequency of ALTRs in Asymptomatic Patients With MoM THA. ALTRs have been described in asymptomatic patients with resurfacing arthroplasties. Whether this concerning finding applies to modular MOM THAs is reported.
Last modified: Thu, 30 Jan 2014 10:48:10 GMT
2013 National Joint Registry
The 2013 National Joint Registry has been released. Data from more than 1.4 million procedures are now registered on the NJR, with 2012/13 the first year for reporting hip, knee, elbow and shoulder data.
Last modified: Tue, 28 Jan 2014 14:56:56 GMT
Hip replacement death rates show 'dramatic fall'
Death rates following hip replacement surgery fell by half in England and Wales between 2003 and 2011, a study in The Lancet has found.
Last modified: Tue, 28 Jan 2014 15:03:58 GMT
FDA requires tracking codes on medical implants-200x39.JPG)
Federal health regulators will begin tracking millions of medical devices, from pacemakers to hip replacements, using a new electronic system designed to protect patients by catching problematic implants earlier.
Last modified: Tue, 28 Jan 2014 14:12:14 GMT
The transparency agenda and the orthopaedic data revolution
Open access article "the transparency agenda and the orthopaedic data revolution". The data revolution is well underway in orthopaedic and trauma surgery.
Last modified: Tue, 28 Jan 2014 13:54:48 GMT
The outcome of the Birmingham Hip Resurfacing in patients aged < 50 years
The outcome of the Birmingham Hip Resurfacing (BHR) in patients aged < 50 years up to 14 years post-operatively (Bone and Joint Journal)
Last modified: Tue, 28 Jan 2014 13:58:05 GMT
Metal Ion Concentrations in Body Fluids after Implantation of all-Metal Hips
Metal Ion Concentrations in Body Fluids after Implantation of Hip Replacements with Metal-on-Metal Bearing – Systematic Review of Clinical and Epidemiological Studies
Last modified: Thu, 19 Dec 2013 13:45:47 GMT
The Hip Society algorithmic approach to diagnosis and management of metal hips
Patient aftercare important in identifying adverse reactions to metal debris following metal-on-metal arthroplasty
Last modified: Thu, 19 Dec 2013 13:32:54 GMT
Which Medical Device in Conversation with the MHRA
Director of Devices at Medicines & Healthcare products Regulatory Agency (MHRA), John Wilkinson, interview
Last modified: Thu, 19 Dec 2013 13:19:14 GMT
Risk of Cancer in Patients with Metal-on-Metal Hip Replacements 
Metal-on-metal total hip replacements not associated with an increased risk of cancer, open access article
Last modified: Thu, 19 Dec 2013 13:02:31 GMT
Report into people with arthritis' awareness of their rights
Arthritis Research UK sheds new light on the awareness that people with arthritis have of their rights within the NHS Constitution, their need for information and support and their experiences of living with the pain of arthritis
Last modified: Thu, 19 Dec 2013 12:53:31 GMT
Consultant-level outcomes data for orthopaedic surgeons launched
Information is being published on 1,594 orthopaedic surgeons conducting hip and knee replacement surgery for the NHS in England.
Last modified: Thu, 19 Dec 2013 12:00:57 GMT
Press release: New tracking system for high-risk medical devices
MHRA Press release: New tracking system for high-risk medical devices in development as the MHRA responds to review on PIP breast implants scandal.
Last modified: Tue, 04 Feb 2014 13:25:47 GMT
High Prevalence of Adverse Reactions to Metal Debris in Small-headed ASR™ Hips
Adverse reactions to metal debris are common with small-headed ASR™ prostheses.
Last modified: Thu, 19 Dec 2013 10:52:49 GMT
J & J Unit Phasing Out All-Metal Hip Devices
DePuy phasing out metal-on-metal and ceramic-on-metal hips
Last modified: Thu, 19 Dec 2013 10:39:10 GMT
National Joint Registry launches its first-ever eBulletin news service
The National Joint Registry has launched its first eBulletin news service – a monthly email delivering news, events and other updates from the NJR and all other relevant healthcare organisations direct to your inbox.
Last modified: Thu, 19 Dec 2013 09:57:57 GMT
FDA "Information for Patients Who Have Metal-on-Metal Hip Implants"-200x39.JPG)
Metal-on-metal hip implant information (from January 2013)
Last modified: Thu, 19 Dec 2013 09:50:10 GMT
Medical Device Alert: ADEPT® 12/14 modular head hip components
MHRA Medical Device Alert: ADEPT® 12/14 modular head hip components manufactured by Finsbury Orthopaedics Ltd (MDA/2013/010)
Last modified: Wed, 18 Dec 2013 15:21:17 GMT
J & J Loses First Case Over Faulty Hip Implant
New York Times article on ASR metal hip trial outcome
Last modified: Tue, 17 Dec 2013 15:23:54 GMT
J & J to phase out all-metal hips-200x39.JPG)
Johnson and Johnson have announced that that they are phasing out all-metal hip replacements
Last modified: Thu, 19 Dec 2013 10:00:47 GMT
EC criticised for slow response to hip problems
The European Commission has been criticised by the UK's Science and Technology Committee the BBC reports.
Last modified: Wed, 27 Feb 2013 12:18:54 GMT
Telegraph reports on undercover investigation
An undercover investigation by the Telegraph claims to have found severe loopholes in the EU approvals system.
Last modified: Fri, 22 Feb 2013 12:12:25 GMT
Hip resurfacing prone to failure, say doctors
Doctors advise against using hip resurfacings due to unacceptable failure rates.
Last modified: Fri, 22 Feb 2013 12:07:50 GMT
Australian National Joint Replacement Registry
Latest data from the Australian National Joint Replacement Registry
Last modified: Fri, 22 Feb 2013 12:03:08 GMT
Birmingham hip resurfacing at a mean of ten years
Results of a long term study examining the Birmingham Hip Resurfacing prosthesis.
Last modified: Fri, 22 Feb 2013 11:50:59 GMT
Telegraph reports on new European regulations 
The Telegraph reports on the potential new rules being drafted in the wake of the metal-on-metal and PIP scandals
Last modified: Fri, 15 Feb 2013 15:56:30 GMT
Update on the DePuy ASR Hip recall
An update on the DePuy ASR Hip recall
Last modified: Fri, 15 Feb 2013 15:36:49 GMT
J&J said to pay to settle first lawsuits over hips
DePuy are reported to pay to resolve three cases before they were scheduled to go to court.
Last modified: Fri, 15 Feb 2013 15:28:56 GMT
BMJ editorial Devices and Desires
A recent article by deputy editor of the British Medical Journal
Last modified: Fri, 15 Feb 2013 15:19:51 GMT
Research suggests upper limit metal ion levels for metal hip resurfacings
Research into ion levels for chromium and cobalt suggest guideline levels where too high
Last modified: Fri, 15 Feb 2013 15:21:11 GMT
National Joint Registry 2012
This is the 2012 annual report from the National Joint Registry (NJR). The NJR collects information on joint replacement surgery and monitor the performance of joint replacement implants.
Last modified: Mon, 24 Sep 2012 15:01:40 BST
Stryker recall Rejuvenate Modular and ABG II modular-neck hip
Stryker recall their Rejuvenate Modular and ABG II modular-neck hip stems due to concerns over corrosion.
Last modified: Mon, 16 Jul 2012 17:45:14 BST
FDA review of metal on metal hips: documents
The FDA have made available all the documents for their review of metal on metal hip implant failures.
Last modified: Mon, 16 Jul 2012 17:27:23 BST
Commons Select Committee session two online
The UK House of Commons' Select Committee on Science and Technology met yesterday to discuss medical device regulation for the second time.
Last modified: Thu, 14 Jun 2012 16:06:12 BST
Commons Select Committee: transcript available
A transcript is available of the UK House of Commons' Select Committee on Science and Technology, session on medical device regulation at which Tom Joyce gave evidence.
Last modified: Thu, 14 Jun 2012 15:56:19 BST
Commons Select Committee: watch online
The UK House of Commons' Select Committee on Science and Technology met yesterday to discuss medical device regulation. They heard oral evidence from a panel of experts including Tom Joyce.
Last modified: Thu, 24 May 2012 12:20:19 BST
Lessons learnt: preventing future artificial joint failures
A summary from our multi-disciplinary event in April in London is now available online.
Last modified: Thu, 24 May 2012 14:35:54 BST
Patient questionnaire now available
A questionnaire exploring the experiences of people who have had metal-on-metal hip replacements is now available. If you have such an implant, please consider completing this anonymous questionnaire.
Last modified: Tue, 17 Apr 2012 16:43:44 BST
Tom Joyce invited to Commons Select Committee
Tom Joyce has been invited to give oral evidence to the UK Commons Science & Technology Select Committee on the regulation of medical implants.
Last modified: Thu, 17 May 2012 16:53:45 BST
Lessons learnt: preventing future artificial joint failures
Lessons learnt: preventing future artificial joint failures - world-class thought leadership at the Institution of Mechanical Engineers. Tom Joyce leads a ground-breaking event bringing together experts to share their knowledge and insight.
Last modified: Thu, 05 Apr 2012 15:24:14 BST
BBC Newsnight and BMJ Investigate hip failures
Tom Joyce's work on hip joint failure features in a BBC Newsnight and British Medical Journal investigation.
Last modified: Wed, 29 Feb 2012 12:23:01 GMT
Latest data confirms high failure rates
The Lancet publishes “unequivocal evidence” on stemmed MOM implants failure and authors join our call for a ban.
Last modified: Tue, 13 Mar 2012 09:50:59 GMT
MHRA update guidance to metal-on-metal hip patients
The UK regulatory body, the MHRA, has updated its advice to surgeons and patients with certain makes of metal-on-metal hip replacement.
Last modified: Tue, 06 Mar 2012 10:46:04 GMT
British Hip Society calls halt on large head metal on metal hips
The British Hip Society annual meeting 2012 in Manchester agreed to call a halt on surgeons implanting large head metal-on-metal hip replacements, after reviewing evidence presented at its meeting.
Last modified: Wed, 07 Mar 2012 14:29:12 GMT
Tom Joyce calls for caution with large head metal-on-metal hips
In an article in Professional Engineer, Tom Joyce calls on surgeons to stop performing hip replacements using large head metal-on-metal total implants as fears were once again raised about their safety.
Last modified: Fri, 02 Mar 2012 15:54:31 GMT
Hip joint failures on BBC's You and Yours
Following widespread news coverage of problems with ASR hip replacements, the BBC programme 'You and Yours' discusses the issues.
Last modified: Wed, 01 Feb 2012 10:59:51 GMT
Danish Television covers problems with failing hip replacements
Our work investigating the reasons for failed hip joint replacements and investigating the views of patients, featured on the Danish consumer programme 'Kontant' this week.
Last modified: Wed, 01 Feb 2012 10:52:27 GMT
Hip joint replacement work features on Irish TV documentary
Dr Tom Joyce’s work investigating the reasons for failed hip joint replacements featured on the Irish national news documentary “Prime Time” this week.
Last modified: Wed, 01 Feb 2012 10:56:38 GMT
Johnson & Johnson expert miscalculates hip angle
A witness for Johnson & Johnson miscalculated in testifying that a Montana man’s metal hip device failed because of the angle at which it was implanted.
Last modified: Wed, 27 Feb 2013 12:42:50 GMT
Surgeon-designer of ASR was paid $20m
The surgeon, Thomas Schmalzried, also told jurors in state court in Los Angeles yesterday that he made $3.6 million in royalties on the ASR hip. Since 2000, DePuy has paid him $20 million, mostly for work on other devices, Schmalzried said.
Last modified: Wed, 27 Feb 2013 12:54:30 GMT
Johnson & Johnson Toxicologist says metal from hips is harmless
Dennis J. Paustenbach testified yesterday on Johnson and Johnson’s behalf at the first of 10,000 lawsuits to go to trial over the ASR hips, which the company recalled in August 2010.
Last modified: Wed, 27 Feb 2013 13:04:06 GMT
Northern Ireland joints National Joint Registry
The National Joint Registry (NJR) is delighted to announce that Northern Ireland will join the registry as of today.
Last modified: Wed, 27 Feb 2013 13:46:43 GMT
Bloomberg report on the ASR trial 
Bloomberg report on the continuing ASR trial
Last modified: Wed, 27 Feb 2013 20:08:39 GMT
MEPs divided ahead of vote on medical devices
EU vote postponed from July 10 to Sept 18
Last modified: Wed, 07 Aug 2013 16:23:48 BST
EU at crossroads on new medical devices legislation
EU at crossroads on new medical devices legislation
Last modified: Wed, 07 Aug 2013 16:19:52 BST
650 French patients fitted with unauthorised replacement hips
650 French patients fitted with unauthorised replacement hips
Last modified: Wed, 07 Aug 2013 16:23:18 BST
Time is running out in NY to file a suit: File or not?
Time is running out in NY to file a suit: File or not? (Part Seven of x) -Plaintiffs opening remarks-a remarkable summary!
Last modified: Wed, 07 Aug 2013 16:27:44 BST
HSE to order artificial hip review
In Ireland, Health Service Executive "to order major review of all patients fitted with metal-on-metal hips
Last modified: Wed, 07 Aug 2013 16:31:27 BST
Bloomberg reports on a mesh trial in US
Bloomberg reports on a mesh trial in US "Bard Executives Hid Vaginal-Mesh Device Flaw, Lawyer Says"
Last modified: Wed, 07 Aug 2013 16:49:33 BST
Stryker has now spent about $400 million on hip recalls
Stryker has now spent about $400 million on Rejuvenate and ABG II modular-neck hip recalls
Last modified: Wed, 07 Aug 2013 16:52:47 BST
New report into people with arthritis' awareness of their rights within the NHS
New report into people with arthritis' awareness of their rights within the NHS Constitution
Last modified: Wed, 07 Aug 2013 16:55:20 BST
Some metal-on-metal hip replacements require early repeat surgery
From Canada "Some metal-on-metal hip replacements are more likely to require early repeat surgery"
Last modified: Wed, 07 Aug 2013 16:58:47 BST
Metal-on-metal total hip replacements do not increase risk of cancer
Metal-on-metal total hip replacements not associated with an increased risk of cancer, open access article
Last modified: Wed, 07 Aug 2013 17:03:16 BST
Interview with John Wilkinson (MHRA)
Director of Devices at Medicines & Healthcare products Regulatory Agency (MHRA), John Wilkinson, interview
Last modified: Wed, 07 Aug 2013 17:05:38 BST
Interview with John Wilkinson (MHRA)
Director of Devices at Medicines & Healthcare products Regulatory Agency (MHRA), John Wilkinson, interview
Last modified: Wed, 07 Aug 2013 17:05:32 BST
An interesting potential development in knee joint replacement
An interesting potential development in knee joint replacement, which has just received 510k clearance from FDA
Last modified: Wed, 07 Aug 2013 17:30:35 BST
Reuters "Medtronic bone graft has limited benefit, may cause harm: reviews
Reuters "Medtronic bone graft has limited benefit, may cause harm: reviews
Last modified: Wed, 07 Aug 2013 17:32:54 BST
Call for improved safety data on medical implants
Call for improved safety data on medical implants
Last modified: Wed, 07 Aug 2013 17:35:22 BST
DePuy phasing out metal-on-metal and ceramic-on-metal hips
DePuy phasing out metal-on-metal and ceramic-on-metal hips
Last modified: Wed, 07 Aug 2013 17:39:03 BST
Hip agony lawsuit
More than 170 South Africans are to launch a landmark class-action lawsuit in the UK for faulty hip implants.
Last modified: Wed, 07 Aug 2013 17:41:43 BST
BBC News "GlaxoSmithKline accused of market 'abuse' "
BBC News "GlaxoSmithKline accused of market 'abuse'"
Last modified: Wed, 07 Aug 2013 17:44:28 BST
Fury over faulty replacement hips
BBC report on UK govt minister who has two ASR resurfacings implanted 'fury over faulty replacement hips'
Last modified: Wed, 07 Aug 2013 17:46:37 BST
Recall of hip replacement system was the right decision
USA Today also gives the DePuy view "Recall of hip replacement system was the right decision at the right time"
Last modified: Wed, 07 Aug 2013 17:49:24 BST