Photon reactions on ice

Radiation-induced reactions on ice could explain the devleopment of life on Earth
Solar wind, photons, solar flare ions and cosmic rays are constantly being launched from the Sun, and the atmosphere above interplanetary bodies such as comets, asteroids or planetary dust particles, corresponding to a much better vacuum than that generally attainable in laboratories, so it is truly an ultrahigh vacuum laboratory for scientists.
Temperature in interplanetary space is often only several Kelvin so that the small particulates in the solar wind often carry numerous layers of cold condensed gases. The primary physical factors considered important to the evolution of life on a planet include temperature, pressure and radiation. Although radiation can destroy living systems, high fluxes of radiation where not sufficient to stop the origin and early evolution of life the ice conditions of early Earth. It is believed that in the past radiation processes have been, and still are, important in the formation of prebiotic macromolecules and their eventual assembly into living organisms.
The physical and chemical basis for important material removal and alteration processes involves atomic and molecular collisions and electronic excitations on the surface of the material. Upon ion, photon or electron impact on matter, excitation and ionisation are the principal results.The surface science community has studied these type of interactions for quite some time and a detailed understanding of the principles of collisional sputtering as well as electronically-induced sputtering or desorption induced by electronic transitions (DIET) has emerged. DIET processes include photon-stimulated desorption (PSD) and electron-stimulated desorption (ESD) of ions and neutral atoms from surfaces. However, electron and photon irradiation can, in addition to desorption, induce novel chemical processes. It has recently become evident that the study of double photon and/or electron induced chemical reactions in CO2 ice can be easily accessible due to the creation of very long-lived transient states induced by the incident radiation.The discovery and study of such reactions forms a key part of the work undertaken by in this research.
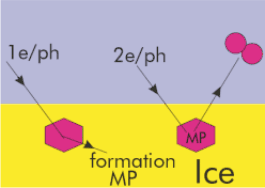
Fig 1: Proposed scheme for production of new molecular species on the icy surfaces of satellites and planets.The scheme involves radiation-induced production of a molecular precursor species (MP), followed by radiationinduced production of new compounds.
In fig 1 a schematic pathway of bi-molecular processes in ice is presented. New chemical precursor states (MP) are formed in ice upon the first incident photon/electron while the new chemical products are desorbed upon the second one.The threshold energies for reactions in CO2 ice may be reduced by more than 7 eV with respect to the gas phase values. Since thick CO2 ice has been detected on the Martian surface, these types of electron induced processes in pure CO2 ice may be important in understanding the evolution of the atmosphere and polar caps of Mars. Previous work also indicates that one should be able to change the outcome of photon/electron induced processes in ice simply by doping the water ice with some substance. For example, in CO2 ice deliberate doping with H2 leads to the formation of formaldehyde.
Stimulated effects in condensed CO2 and mixtures of CO2 with C2H6 ice by means of photons irradiation from a synchrotron source have been studied and monitored by electron photoemission. This work confirmed that after only a few minutes of irradiation with mono-energetic photons, new states in the valence band of pure C2H6 ice are observed (below).This is direct evidence of the formation of new molecular species in the ice upon irradiation. Similarly, time of flight spectra, show that novel species can be detected by ESD, through desorption from the surface of CO2 ice after a period time of irradiation. Such reaction pathways in the gas phase are orders of magnitude less probable.
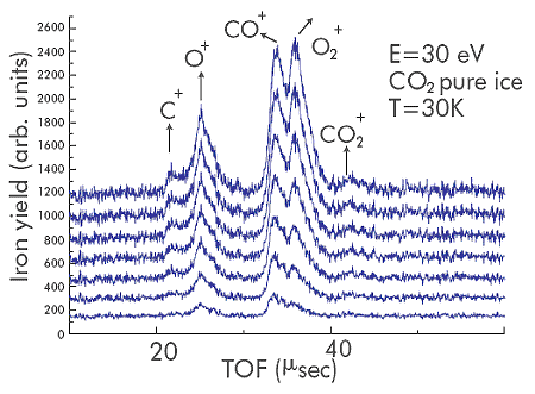
Fig 2: Valence-band spectra (hv= 40eV) collected at normal emission from C2H6 ice condensed at 30 K. Black parts in spectra are differences which occur after 70 min of irradiation with photons.
The experiments described above indicate that a range of molecules could be formed in ice leading to more complex species such as prebiotic macromolecules.This type of work, particularly elucidation of detailed mechanisms and main co-products, is still in the early stages of investigation.
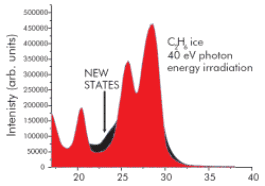
Fig 3: Time-of-flight (TOF) spectra from 15 L of CO2 ice condensed at 30 K; the ions are desorbed due to impact of 30 eV electrons. The peak associated with O2+ rises with time in a different manner from that of the other detected ions (CO+, O+ and C+) which is attributed to the proposed schematic reaction given in fig 1
Many different cocktails of gases and their chemistry at different temperatures, where roughness and phase changes can play a role, may need to be explored in order to mimic early or current atmospheres on the planets. NASA probes have already been launched and are at this moment on their way to satellites such as Titan - which is believed to have an atmosphere similar to that of early Earth and hence may shed light on how life began. Similarly, a mission to Gannymede is planned, which has active volcanoes and considerable oxygen and water in the atmosphere, so data will be required from the wider science community in order to explain the results obtained by these probes. In particular, one needs to know molecular and ion yields, ratios, thresholds and optical properties of iced surfaces as well as surface chemistry on ‘cocktails’ of condensed gases such as those mentioned here.
Publications
- Šiller L, Sieger MT, Orlando TM
Electron-stimulated desorption of D2O coadsorbed with CO2 ice at VUV and EUV energies
JOURNAL OF CHEMICAL PHYSICS 118 (19): 8898-8904 MAY 15 2003

