Gold Nitride

Binary nitrides exhibit a remarkable variety of desirable characteristics such as high melting points, high hardness and a robust chemical stability which makes them useful coating materials. Gold is particularly favoured in the electro-plating of electrical components due to its high conductivity and robustness in the ambient atmosphere which is often improved by combining the parent material with iron, nickel or cobalt.
Gold films incorporating gold nitride are 50 % harder than those without thus gold nitride should provide a better material for contact coatings in electronic circuits. For almost twenty years researchers had tried to synthesize gold nitride but with no success until a method, discovered at Newcastle, created gold nitride by implanting low-energy nitrogen ions into a single Au(110) crystal surface with plasma and reactive ion sputtering.
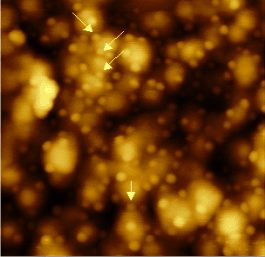
1 x 1 µm AFM image of gold nitride after plasma etching, the arrows show large clusters covered by smaller structures.
N1s photoemission spectra (below) of Au(110) irradiated with 500 eV nitrogen ions at 5770 and 21 700 μC/cm2 doses show two peaks at 396.6±0.2 eV (A) and 402.7±0.2 eV (B).
Peak A, at 396.6 ± 0.2 eV lies close to the binding energies for chemisorbed nitrogen on Cu(110) of 396.5–396.8 eV (depending on coverage) while peak B corresponds to nitrogen deeper in the crystal. Peak A corresponds to a surface AuxN phase and is the first direct observation of a gold nitride.
This technique, which attracted significant national and international media attention, offers the possibility of producing gold nitride at commercial scales to be used in place of more expensive gold coatings with significant environmental benefits.
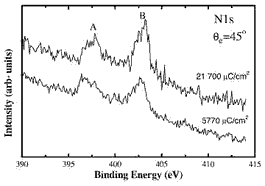
Photoemission spectra obtained for nitrogen ion doses of 5770 and 21 700 μC/cm2 at 300 K - peak (A) shows the first direct evidence of gold nitride
Results
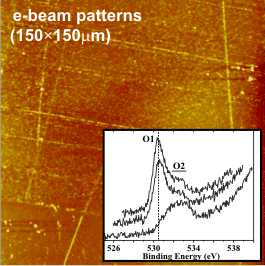
Electron beam patterns on a gold film containing gold nitride. Oxygen photoemission spectra as irradiation progresses shown inset
Ongoing research is directed towards the further study and characterization of this new material and already significant results have been published.
Recent work has shown how nitrogen species in gold nitride films decompose under x-ray irradiation. This could allow patterns to be written directly into gold nitride surfaces through electron beam or photon based lithographic techniques and would open up its potential uses in industrial applications.
Our Publications
- Siller L, Alves L, Brieva AC, Butenko YV, Hunt MRC
Gold Nitride: Preparation and Properties
TOPICS IN CATALYSIS 52 (11): 1604-1610 OCT 2009 - Brieva AC, Alves L, Krishnamurthy S, Siller L
Gold surface with gold nitride–a surface enhanced Raman scattering active substrate
JOURNAL OF APPLIED PHYSICS 105 Art. No. 054302 MAR 2009 - Alves L, Hase TPA, Hunt MRC, Brieva AC, Siller L
X-ray diffraction study of gold nitride films: Observation of a solid solution phase
JOURNAL OF APPLIED PHYSICS 104 Art No. 113527 DEC 2008 - Butenko YV, Alves L, Brieva AC, et al.
X-ray induced decomposition of gold nitride
CHEMICAL PHYSICS LETTERS 430 (1-3): 89-92 OCT 19 2006 - Šiller L, Hunt MRC, Brown JW, et al.
Nitrogen ion irradiation of Au(110): formation of gold nitride
SURFACE SCIENCE 513 (1): 78-82 JUL 10 2002
- Krishnamurthy S, Montalti M, Wardle MG, et al.
Nitrogen ion irradiation of Au(110): Photoemission spectroscopy and possible crystal structures of gold nitride
PHYSICAL REVIEW B 70 (4): Art. No. 045414 JUL 2004
- Šiller L, Peltekis N, Krishnamurthy S, et al.
Gold film with gold nitride - A conductor but harder than gold
APPLIED PHYSICS LETTERS 86 (22): Art. No. 221912 MAY 30 2005
- Butenko YV, Alves L, Brieva AC, et al.
X-ray induced decomposition of gold nitride
CHEMICAL PHYSICS LETTERS 430 (1-3): 89-92 OCT 19 2006
Related Links
Scientists create gold substitute - BBC News October 2003

