Development of CDK9 inhibitors
CDK9 is a validated target for the treatment of cancer, cardiac hypertrophy, and human immunodeficiency virus. Inhibition of CDK9 selectively reduces the transcription of short-lived mRNAs, including those that encode regulators of proliferation and apoptosis such as c-Myc and Mcl-1. These proteins are upregulated in many cancers, leading to the inhibition of apoptosis and the promotion of proliferation. The therapeutic potential of interfering with these processes has been highlighted by promising preclinical and clinical data gathered for inhibitors of CDK9 and its regulators. Structural studies can distinguish unique features of its active site amenable to exploitation to aid the development of selective CDK9 inhibitors. One notable feature is that the CDK9 active site is more malleable than that of CDK2 so that it can more readily accommodate large and flexible compounds.
Highlights
- CDK9 binding of members of a 4- (thiazol-5-yl)-2-(phenylamino)pyrimidine-5-carbonitrile series results from the relative malleability of the CDK9 active site rather than from the formation of specific polar contacts
- CAN508 binding reveals CDK9-specific conformational changes and identifies a CDK9-specific hydrophobic pocket, adjacent to the C-helix
- DRB binding suggests the possibility to exploit halogen atoms in inhibitor design to specifically target CDK9
- Structure determination of CCT068127, a potent CDK2 and CDK9 inhibitor
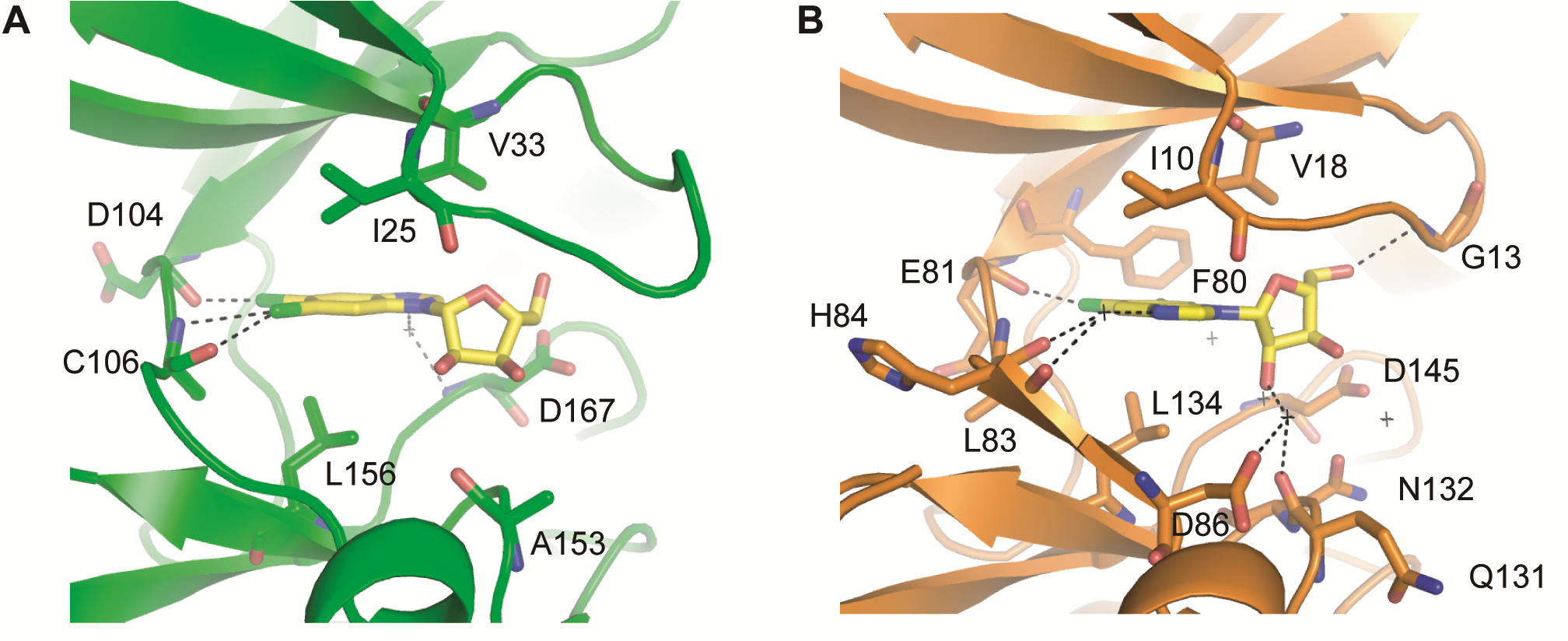
Potent CDK9 inhibitors bound to CDK9 or CDK2. (A) Compound 12u binding to CDK9 (B) DRB binding to CDK9 (C) CCT068127 binding to CDK2.
Collaborators: Professor Shudong Wang, University of South Australia; Professor Peter Fischer, University of Nottingham; Dr Aude Echalier-Glazer, Exscientia; Professors Paul Workman, Michelle Garrett and Julian Blagg, ICR, and colleagues at NICR.
Associated publications:
Molecular profiling and combinatorial activity of CCT068127: a potent CDK2 and CDK9 inhibitor, (2018) Whittaker et al., Mol Oncol 12:287-304
Comparative Structural and Functional Studies of 4‐(Thiazol-5-yl)-2- (phenylamino)pyrimidine-5-carbonitrile CDK9 Inhibitors Suggest the Basis for Isotype Selectivity, (2013) Hole et al., J. Med. Chem. 56: 660−670.
Substituted 4-(thiazol-5-yl)-2-(phenylamino)pyrimidines are highly active CDK9 inhibitors: synthesis, X-ray crystal structures, structure-activity relationship, and anticancer activities, (2013)
Shao et al., J Med Chem. 56:640-59.
The CDK9 C-helix exhibits conformational plasticity that may explain the selectivity of CAN508, (2012) Baumli et al., ACS Chem. Biol. 7: 811 816.
Halogen bonds form the basis for selective P-TEFb inhibition by DRB, (2010), Baumli et al., Chem Biol. 17:931-6.


