CDK1-cyclin B bound to ATP-competitive inhibitors
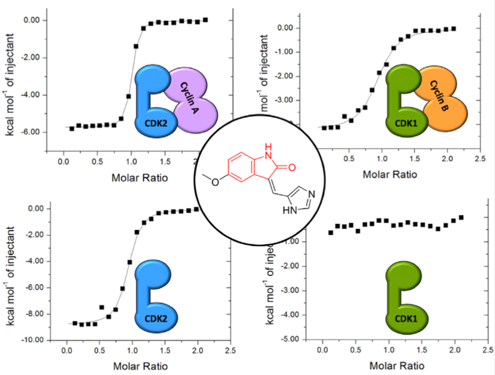
As it is an essential protein, the selection of CDK1 as a potential drug target may appear counter-intuitive. However, with appropriate selection of molecular context, its unique ability to phosphorylate specific substrates may offer opportunities for therapeutic exploitation. In this context, potent and selective tool compounds are required to validate CDK1 as a clinical target. A challenge for drug design is to identify compounds that are able to selectively distinguish CDK1 and CDK2 as these CDKs share considerable sequence identity, and the residues that line their ATP binding sites are identical or highly conserved. CDK1 has a malleable fold that responds to cyclin and ATP-competitive inhibitor binding. We have used a range of biophysical techniques to compare the CDK1 and CDK2 folds in the cyclin-free and cyclin-bound states. We have also determined a number of CDK1-cyclin B-inhibitor complex structures to elaborate the changes that accompany inhibitor binding as a starting point for inhibitor design.
Highlights
- The first structures of ATP-competitive inhibitors bound to CDK1-cyclin B
- Demonstration that ATP-competitive inhibitors distinguish cyclin-free CDK1 from CDK2
- Determination of the first cyclin-free CDK1-ATP inhibitor complex structures
- Targeting cyclin-free CDFs may offer a clear selectivity window over CDK1
Collaborators: Colleagues at the NICR
PDB entries: 4Y72
Associated publications:
CDK1 structures reveal conserved and unique features of the essential cell cycle CDK, (2015) Brown et al., Nature Communs 6:6769.
Differences in the Conformational Energy Landscape of CDK1 and CDK2 Suggest a Mechanism for Achieving Selective CDK Inhibition, (2019) Wood et al., Cell Chem Biol. 26:121-130.


