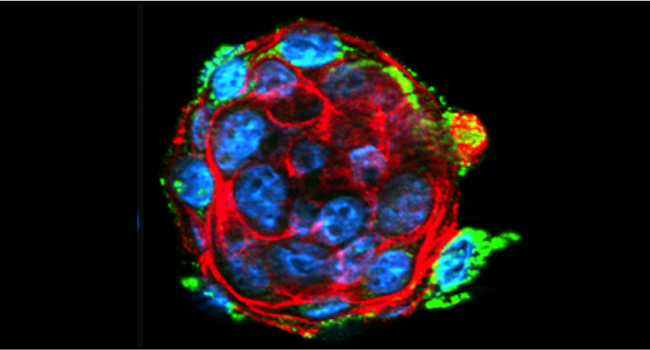
Keratinocyte stem cells
Studying the impact of mechanical signalling on keratinocyte stem cells in health and disease
Keratinocyte stem cells (KSC) regulate epidermal homoeostasis. Wounding stimulates KSC to exit their niche, proliferate and participate in wound closure. KSC are also activated in hyperproliferative psoriatic epidermis and psoriasis may be provoked by mechanical trauma to skin (Koebner phenomenon). The signaling mechanisms involved in activating KSCs are not well characterized.
Keratinocytes are able to sense mechanical strain and transform it into biochemical signals. Mechanical signal transduction implies an intact cytoskeleton as well as cell-cell and cell-matrix contacts. The literature on mechanical signalling in keratinocytes is based on results using differentiated keratinocytes whereas the response of KSC has not yet been studied. Studies involving other types of stem cells support the hypothesis that mechanical load regulates self-renewal, lineage commitment and migration of stem cells.
Lee Wallace’s (PhD student from Oct/2010-Sept/2013) tested the hypothesis that mechanical stress regulates KSC function in a 3D context, relevant to wound healing. Lee utilized a 3D keratinocyte culture system which allows the keratinocytes to form a cytoskeleton and adhesion structures which are essential for mechanosignalling. Using keratinocyte clusters in combination with the “Flexcell” system, Lee found that the response of KSC to stretch differs from the response of differentiated keratinocytes (Wallace and Reichelt, 2013). Further results will be published soon.
Work on this project has been supported by the Institute of Cellular Medicine and the Medical Faculty at Newcastle University, and NESCI.