Research Lay Summaries
Keratinocyte stem cells
Forcing stem cells out of their niche to improve wound healing
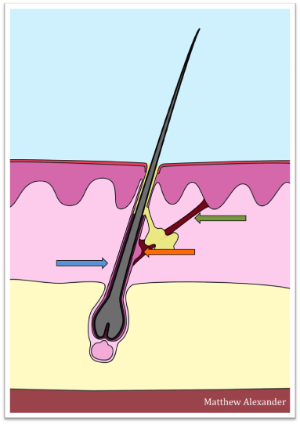
Skin stem cells are responsible for constant skin renewal and wound healing. There is one population of extremely potent stem cells sitting in a niche (orange arrow) at the interface between the hair follicle (blue arrow) and a small muscle (green arrow) the contraction of which causes the hair to stand on end when we are cold. It is known from other types of stem cells in our bodies, such as bone stem cells, that they are activated by force. Our group is investigating whether contraction of the hair follicle muscle resulting in stretching of the niche stem cells activates this cell population. If we find that skin stem cells are activated by stretch we will explore whether this mechanism may be harnessed to accelerate healing of difficult wounds.
Psoriasis
Defining novel therapeutic targets by understanding how mechanical stress contributes to psoriasis progression
Psoriatic plaques are characterised by a strong thickening of the skin. This thickening is due to excessive proliferation of cells (keratinocytes) which constitute the epidermis, the uppermost layer of the skin. The cause for the increase in keratinocyte proliferation and hence the development of psoriatic plaques is unknown. A common observation however, is that psoriatic plaques tend to localize to the knees and elbows, areas that are particularly subject to mechanical stress resulting from the action of bending. Moreover, plaques often develop at sites of trauma or injury. It is well-established that most tissues react with increased growth following mechanical stress. Some of the molecular signals for this phenomenon are known and interestingly these are also activated in psoriatic skin.
The Newcastle team is currently testing the hypothesis that psoriatic skin and particularly the epidermal keratinocytes are oversensitive to mechanical stress. Evaluation of the signals regulating the development of psoriatic plaques may lead to the definition of novel therapeutic targets for psoriasis treatment.
Gene therapy
A therapy for inherited blistering skin disorders
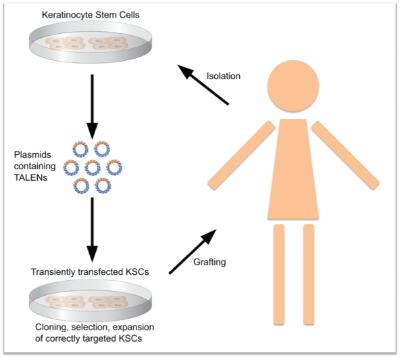
Our research team is currently developing gene therapies for two inherited blistering skin diseases: epidermolysis bullosa simplex (EBS) and epidermolytic ichthyosis (EI). The aim is to use ‘molecular scissors’ (novel tools called ‘TALENs’) to inactivate or repair the disease-causing genes in skin cells from patients. As the ‘molecular scissors' cannot be applied to the skin directly we will treat skin cells, derived from patient biopsies, in the laboratory and transplant successfully corrected cells back to the patient’s skin. The ‘molecular scissors’ will cause a permanent correction of the gene defects in the patient’s skin cells and will disappear without leaving any traces within the cells. The grafted cells will support regeneration of a healthy resilient skin.
Developing a test system for gene therapies for inherited skin disorders
Developing a test system for gene therapies for inherited skin disorders
In order to validate the gene therapy approaches currently being developed by our research group we are establishing a 3D cell culture model of EBS and EI skin which can serve as simple test systems that can be used prior to the first in-man studies.
For the 3D model we are using skin cells carrying defective keratin genes which are similar to the disease-causing genes found in patients. These cells are grown in culture dishes in the laboratory into 3D sheets that look like the uppermost layer of the skin. This uppermost skin layer (epidermis) is the layer that is affected in patients with EBS and EI; and the 3D sheets grown from diseased cells show the same features as seen in the patient’s skin. After successful destruction of the defective keratin genes using ‘molecular scissors’ the 3D sheets grown from these skin cells should lose the disease features and look like sheets grown from healthy skin cells.
Keratins
Skin resilience relies on the keratin scaffold within its cells – but how does that work exactly?
Keratins are found in various cell types in our bodies, such as liver cells or skin cells. In the cells of the top layer of the skin (epidermis) keratins are the most prominent proteins. The function of these cells, called keratinocytes, is to form the barrier of the skin which on the one hand needs to be soft to provide sufficient flexibility to allow movement and on the other hand needs to be resilient enough to protect us from environmental challenges such as trauma. Keratins are essential for the function of keratinocytes building a scaffold which serves as the cell’s ‘skeleton’. The importance of the keratin scaffold for the stability of the skin becomes obvious in patients with epidermolysis bullosa simplex (EBS) who carry mutations in keratins. Although the mutant keratins can still form a cell’s ‘skeleton’ it is extremely fragile and cannot withstand stress very well resulting in blistering and skin coming off upon the mildest touch. There is no cure for these inherited diseases and our team is currently developing a gene therapy for EBS.
There are more than 11 varieties of keratins present within distinct cell types in the epidermis but their specific roles within the cell’s skeleton and for the stability of the skin are unknown. One of our projects addresses this question by investigating the response to stretching of keratinocytes grown in the lab and engineered to contain ‘skeletons’ composed of different sets of keratins.
