Success Stories
DNA-PK inhibitors
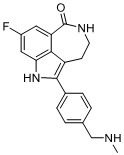
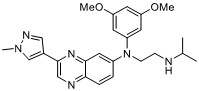
The Newcastle team undertook pioneering work to identify inhibitors of DNA-dependent protein kinase (DNA-PK), such as NU7441 (currently used as a tool compound in 95 publications). A collaboration with AstraZeneca revealed the more selective NU5455 which was not taken forward as a clinical compound. As part of this collaboration, an anilinopurinone-derived inhibitor series was identified from the AstraZeneca compound collection, and was initially pursued as a joint project. Subsequently, AstraZeneca independently optimised the series and declared AZD7648 as a clinical candidate. The compound entered clinical development in October 2019, with the aim of examining its activity in cancer patients when combined with either pegylated liposomal doxorubicin or the PARP-inhibitor olaparib.