News
New Paper – Surfactant maps to optimize key reactions in DEL synthesis
Org. Biomol. Chem., 2025, 23, 6745-6754
DOI: 10.1039/D5OB00864F
https://pubs.rsc.org/en/content/articlelanding/2025/ob/d5ob00864f
Jake A. Odger, Matthew J. Anderson, Thomas P. Carton, Bao Nguyen, Kevin Foote and Michael J. Waring*
DNA-encoded libraries (DELs) are an emerging technology and are increasingly important in hit identification at the early stage of the drug discovery process. DELs consist of a vast number of organic molecules, with each member covalently attached to a specific DNA sequence, which functions as a unique, amplifiable barcode. Libraries are commonly synthesised through the use of combinatorial split-and-pool methodology and this technique couples the conjugation of chemical building blocks with the enzymatic ligation of an encoding DNA oligomer, whereby each DNA sequence is specific to the chemical monomer it encodes.
Many reactions employed for library synthesis are inefficient and result in significant DNA-damage, incomplete conversion and the formation of side products, which compromise the fidelity of the resulting library. Conventionally, DEL reactions should be carried out in aqueous media at high dilutions, avoid damaging the integrity of the DNA barcode, proceed with high yields and afford little to no side products.
The use of surfactants in DEL syntheses has been shown to be advantageous and we have previously developed a wide array of reactions that are promoted by the micelle-forming surfactant TPGS-750-M. It is theorised that micellar structures localise organic reagents within the hydrophobic core of the micelles, whilst the DNA barcode remains in the aqueous compartment.
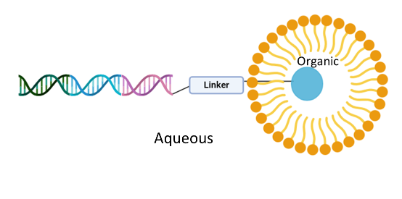 In this paper we demonstrate further improvements to three established on-DNA micelle-mediated reactions (Suzuki–Miyaura coupling, reductive amination and amide coupling) that have been improved to afford greater conversions to desired product across a broad substrate scope through the implementation of alternative surfactants to catalyse the reaction. No damage to the duplex DNA tags was detected in these reactions, even with the use of ionic surfactants and the surfactant map was demonstrated to be an effective tool for surfactant selection and optimisation in micellar catalysis.
In this paper we demonstrate further improvements to three established on-DNA micelle-mediated reactions (Suzuki–Miyaura coupling, reductive amination and amide coupling) that have been improved to afford greater conversions to desired product across a broad substrate scope through the implementation of alternative surfactants to catalyse the reaction. No damage to the duplex DNA tags was detected in these reactions, even with the use of ionic surfactants and the surfactant map was demonstrated to be an effective tool for surfactant selection and optimisation in micellar catalysis.
Finally, a representative DNA-conjugated compound was successfully synthesised using the optimal surfactant for each of the investigated reactions, and PCR amplification followed by next-generation sequencing (NGS) confirmed that the conditions did not cause a detrimental effect to the DNA barcode integrity, highlighting applicability to library synthesis.
Last modified: Thu, 31 Jul 2025 20:35:37 BST