News
New Paper – Micellar Reductive Aminations for DELs
Chem. Eur. J., 2024, e202400239
https://doi.org/10.1002/chem.202400239
https://chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/chem.202400239
Matthew J. Anderson, Thomas P. Carton, Catherine L. A. Salvini, James J. Crawford, Garry Pairaudeau and Michael J. Waring*
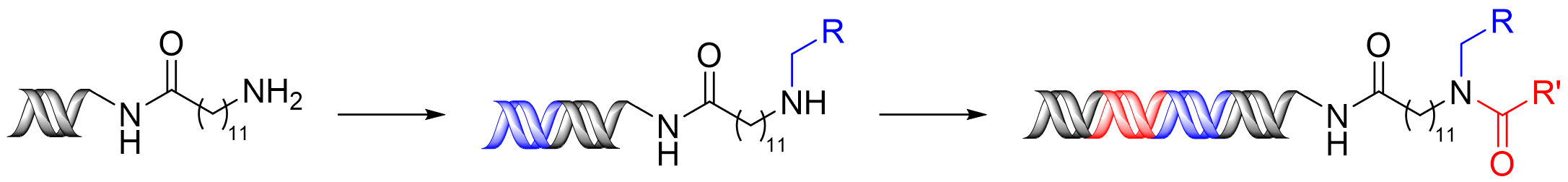
The emergence of DNA-encoded libraries (DELs) as a leading technology for the identification of hit molecules in medicinal chemistry and chemical biology has had a positive impact in drug discovery as large, diverse libraries can be readily generated. DELs comprise large collections of organic molecules attached to a complementary DNA sequence, which serves as an identifiable barcode unique to each compound. Such DELs are commonly synthesised via split-and-pool methodology. Despite many advantages, limitations persist in the chemistries applicable to on-DNA synthesis as reactions require aqueous conditions at high dilutions and milder reagents to limit damage to the DNA barcode.
Many pharmaceutical compounds incorporate multiple C-N bonds, over a quarter of which are synthesised via reductive aminations. However, few on-DNA reductive amination procedures have been developed. Described in this new paper is the highly efficient application of the micelle-forming surfactant, TPGS-750-M, to the on-DNA reductive amination of DNA-conjugated amines. This methodology exhibits a broad substrate scope and functional group compatibility, including the coupling of a range of benzylic, heteroaromatic and aliphatic aldehydes with high efficiency. Transferability across a series of alternative primary amines has also been effectively demonstrated, alongside the development of a modified procedure that is applicable for the coupling of DNA-tagged secondary amines. This allows simple access to diverse secondary and tertiary amine scaffolds and resultant branched structures that are currently rare in the literature. The procedure is compatible with DNA amplification and sequencing, demonstrating its applicability to DEL synthesis.
In this case, the application of micellar catalysis has proven to be critical to promoting greater reaction efficiency. Overall, the broad substrate scope, alongside the highlighted compatibility with DEL synthesis, underlines the value of this methodology for the development of increasingly diverse and effective DELs.
Last modified: Tue, 23 Jan 2024 08:34:10 GMT