News
New Paper – Expanding the scope of Suzuki−Miyaura cross-couplings for DELs
J. Org. Chem. 2021, 86, 17930-17935
https://doi.org/10.1021/acs.joc.1c02259
https://pubs.acs.org/doi/10.1021/acs.joc.1c02259?ref=pdf
James H. Hunter, Marco Potowski, Harriet A. Stanway-Gordon, Andrew Madin, Garry Pairaudeau, Andreas Brunschweiger* and Michael J. Waring*
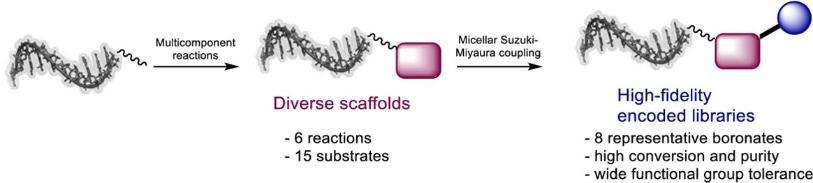
Progress in method development for diverse encoded screening library design gives access to a broad spectrum of DNA-coupled (hetero)aryl halides. In the application to DELs, these compounds are synthesised as complex mixtures and therefore require synthetic methods that are robust and broadly applicable. Diverse encoded compound mixtures will display a variety of steric and electronic effects that may affect the reaction outcome, and they may contain functional groups that are potential catalyst poisons and/or possess functionality that could give rise to side reactions or degradation under the reaction conditions.
In collaboration with the Brunschweiger group, who prepared the substrates using their multicomponent reactions, this paper describes an exceptionally varied scope of the micellar-promoted Suzuki−Miyaura coupling reaction for on-DNA substrates. The reaction is compatible with a widely diverse array of aryl bromides and iodides across a range of boronate-coupling partners. Perhaps most importantly, the reaction tolerates all of the functionality that would be desirable in a drug-like DEL. We have previously demonstrated the scope of the reaction with respect to the off-DNA boronates and the compatibility with encoded library synthesis. Together, the methodology is highly effective for producing high-fidelity DELs. The combination of the robustness of this transformation with the diverse scaffolds prepared using multi-component reactions employed in this work will lead to DELs of exceptional structural diversity.
Last modified: Mon, 19 Dec 2022 17:17:34 GMT