News
New Paper – Discovery of ERK5 Kinase Domain Inhibitors
J. Med. Chem. 2022, 65, 6513-6540
https://doi.org/10.1021/acs.jmedchem.1c01756
https://pubs.acs.org/doi/10.1021/acs.jmedchem.1c01756
Duncan C. Miller, Tristan Reuillon, Lauren Molyneux, Timothy Blackburn, Simon J. Cook, Noel Edwards, Jane A. Endicott, Bernard T. Golding, Roger J. Griffin, Ian Hardcastle, Suzannah J. Harnor, Amy Heptinstall, Pamela Lochhead, Mathew P. Martin, Nick C. Martin, Stephanie Myers, David R. Newell, Richard A. Noble, Nicole Phillips, Laurent Rigoreau, Huw Thomas, Julie A. Tucker, Lan-Zhen Wang, Michael J. Waring, Ai-Ching Wong, Stephen R. Wedge, Martin E. M. Noble and Celine Cano*
The non-classical extracellular signal-related kinase 5 (ERK5) mitogen-activated protein kinase pathway has been implicated in increased cellular proliferation, migration, survival and angiogenesis; presenting ERK5 inhibition as an attractive approach for cancer treatment. Selective ERK5 kinase inhibitors may be useful in elucidating the role of this signalling protein in cancer; however, the development of such inhibitors has been challenging.
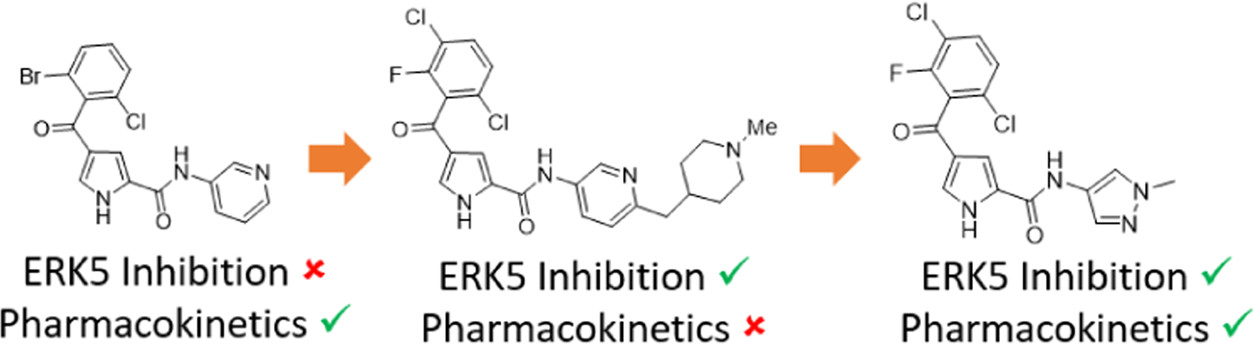
Previously, we described the identification of selective pyrrole carboxamide-based ERK5 inhibitors with sub-micromolar ERK5 kinase activity. To identify a tool compound for target validation studies from this series, improvement in ERK5 inhibitory potency while maintaining the attractive pharmacokinetic properties and selectivity profile was required.
This new paper describes parallel optimisation of potency and ADME properties to identify a non-basic pyrazole compound with optimal balance of ERK5 inhibition and oral exposure.
Introduction of small lipophilic substituents at the 3-position of the benzoyl group of pyrrole carboxamide ERK5 inhibitors led to improved inhibition. Appending a basic centre to the heteroaromatic amide substituent provided nanomolar inhibitors. However, the more potent basic analogues suffered from high efflux ratios in the caco-2 membrane permeability assay that translated to low oral bioavailability in vivo. Smaller, non-basic pyrazole provided the best balance of potency and in vitro ADME properties and had good oral bioavailability in mouse.
Last modified: Mon, 19 Dec 2022 17:15:28 GMT