News
New Paper – Covalent targeting of an alternative EGFR cysteine
J. Med. Chem. 2025, XXXX, XXX, XXX-XXX
https://doi.org/10.1021/acs.jmedchem.5c02924
https://pubs.acs.org/doi/10.1021/acs.jmedchem.5c02924
Hannah L. Stewart, Cinzia Bordoni, Claire E. Jennings, Islam Al-Khawaldeh, Mathew P. Martin, Richard A. Noble, Nicole Phillips, Sara Pintar, Lisa Prendergast, Huw D. Thomas, Lan-Z. Wang, Jessica E. Watt, Anita Wittner, Agnieszka K. Bronowska, Céline Cano, Martin E. M. Noble, Stephen R. Wedge, and Michael J. Waring*
Epidermal growth factor receptor (EGFR) is a tyrosine kinase that drives cell proliferation and survival when activated and oncogenic EGFR mutations are a major cause of nonsmall cell lung cancer (NSCLC).
First-generation EGFR inhibitors (e.g. erlotinib, gefitinib) are clinically effective in lung cancer patients with the Exon19del or L858R mutations, but resistance usually develops within a year, most often via mutation of the “gatekeeper” residue (T790M). Second-generation covalent inhibitors (e.g. afatinib and dacomitinib) have increased affinity through the covalent interaction but lack sufficient selectivity for mutant over wild-type EGFR. Third-generation inhibitors, especially osimertinib, were designed to target T790M mutants while retaining wild-type selectivity and are now standard therapy.
Despite these advances, resistance to third-generation inhibitors inevitably emerges, commonly through mutation of C797, which prevents covalent binding. Therefore, there is an urgent need for new treatments for osimertinib-resistant EGFR mutants that retain the advantages of the covalent mechanism.
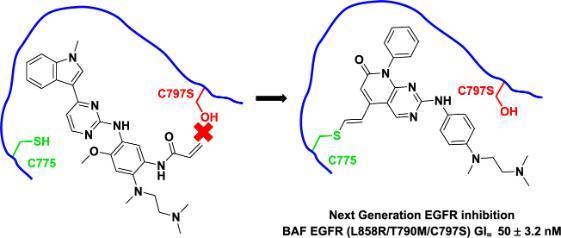
In this paper, the authors identified an alternative cysteine residue (C775) in the EGFR ATP-binding pocket that they reasoned could be a potential new covalent target. Although this residue proved more challenging to access and had been minimally explored previously.
Compounds were designed and synthesised to inhibit EGFR exclusively through covalent modification of C775, with selectivity over wild-type EGFR. Optimisation of the alkynylpyridopyrimidinone scaffold that was discovered led to potent compounds which demonstrated inhibition of EGFR phosphorylation and tumour growth in all EGFR mutant cell lines. The covalent C775 mode-of-action was comprehensively established.
Last modified: Tue, 23 Dec 2025 18:17:41 GMT