News
New Paper – Conformational studies of N-alkyl-N’-aryl ureas
Bioorg. Med. Chem., 2023, 117387
https://doi.org/10.1016/j.bmc.2023.117387
https://www.sciencedirect.com/science/article/pii/S0968089623002353?via%3Dihub
Hannah L. Stewart, Marta Bon, Corinne Wills, Mathew P. Martin, Lan Z. Wang, Eilidh S. Mackenzie, Paul G. Waddell, Michael J. Waring*
As well as being an important functional group in small molecule drugs, ureas have wider applications in organic chemistry. The urea has the potential to form a number of stable hydrogen bonds and understanding of their conformation is of critical importance for rational design of urea-containing bioactive compounds.
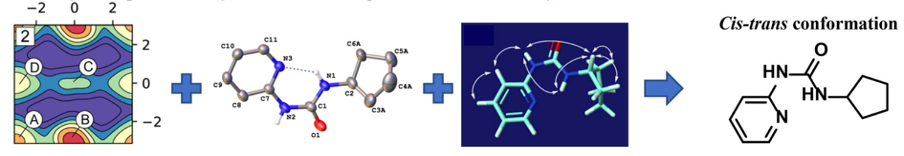
Substituted ureas can adopt four main conformations, classified by the orientation of the large N- and N’-substituents, which can be: trans-trans, cis-trans, trans-cis or cis-cis relative to each other. When considering the incorporation of the urea group within new drug discovery compounds, an understanding of the interplay between these conformations is essential. Whilst the conformational preferences of biaryl ureas have been extensively studied, very little attention has been paid to alkylated analogues.
In this paper we describe a systematic study of N-aryl (phenyl and pyridyl)-N’-cyclopentyl ureas with differing N-methylation patterns using Well Tempered Metadynamics at a semi-empirical level in implicit water (GBSA) using Well-Tempered Metadynamics to generate their conformational free-energy landscapes. Geometries and energetics of the most relevant configurations are further refined using DFT level of theory. Validation for the computation was obtained via synthesis of all analogues, followed by conformational studies by X-ray crystallography and NMR.
Findings reveal that the methylation pattern significantly affects the conformational preference of the system. Notably, N-phenyl-N’-cyclopentyl urea is shown to adopt both the trans-trans, and cis-trans conformations with equal energy and that the cis-trans conformation can be significantly stabilised by the presence of an internal hydrogen bond to the N’-hydrogen.
To our knowledge, this is the first study into the low energy conformations of N-alkyl-N’-aryl ureas. Not only will this inform the design of drugs incorporating this motif, but it will also give useful conformational information for use in wider applications.
Last modified: Wed, 21 Jun 2023 20:42:36 BST