News
New Paper - New developments in the chemistry for DNA-encoded library synthesis
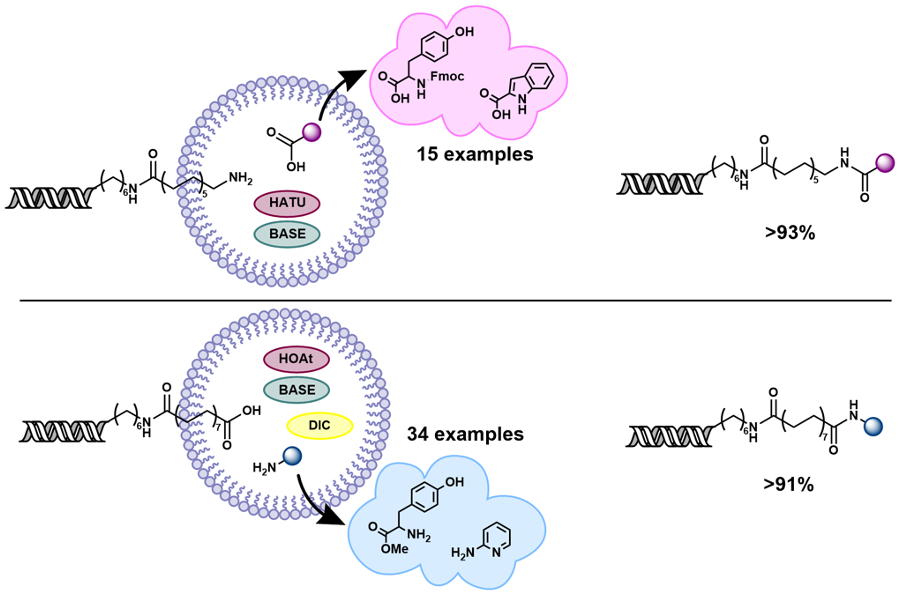
Highly efficient on-DNA amide couplings promoted by micelle forming surfactants for the synthesis of DNA encoded libraries.
Chem. Sci., 2021, 12, 9475-9484
https://doi.org/10.1039/D1SC03007H
https://pubs.rsc.org/en/content/articlelanding/2021/sc/...
James H. Hunter, Matthew J. Anderson, Isaline F. S. F. Castan, Jessica S. Graham, Catherine L. A. Salvini, Harriet A. Stanway-Gordon, James J. Crawford, Andrew Madin, Garry Pairaudeau, Michael J. Waring*
The methods developed here provide a highly efficient and generally applicable synthesis for on-DNA amide coupling. This will be of great utility in the preparation of high fidelity DELs, especially those based on peptides and drug-like small molecules.
The benefit of the application of micellar technology to DELs is demonstrated clearly by this work. The enhancement in both reaction conversion and product purity using micelle forming surfactants in DEL synthesis will be of significant benefit to the field. The combination of more hydrophobic linkers with micellar conditions demonstrates an additional improvement in reaction efficiency and provides evidence that reactions can be improved by increasing the affinity of DNA-linked substrates for hydrophobic micelles.
The ability to carry out efficient amide couplings, the most commonly used reaction in DEL synthesis and medicinal chemistry more generally, will lead to a large number of higher quality DELs with wide substrate scope. The development of an efficient method for N-to-C on-DNA coupling allows the synthesis of peptide-type libraries from simple amino esters, removing the need for Fmoc-protected amino acids that are required for C-to-N synthesis, thus greatly enhancing the accessible scope of this type of library.
Last modified: Mon, 19 Dec 2022 17:22:09 GMT