Hsp90-Cdc37 Chaperone System
Differential Regulation of G1 CDK Complexes by the Hsp90-Cdc37 Chaperone System
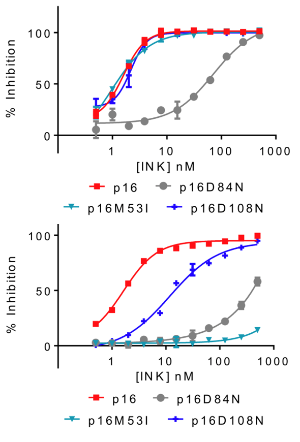
Selective recruitment of protein kinases to the Hsp90 system is mediated by the adaptor co-chaperone Cdc37. We show that assembly of CDK4 and CDK6 into protein complexes is differentially regulated by the Cdc37-Hsp90 system. Like other Hsp90 kinase clients, binding of CDK4/6 to Cdc37 is blocked by ATP-competitive inhibitors. Cdc37-Hsp90 relinquishes CDK6 to D3- and virus-type cyclins and to INK family CDK inhibitors, whereas CDK4 is relinquished to INKs but less readily to cyclins. p21CIP1 and p27KIP1 CDK inhibitors are less potent than the INKs at displacing CDK4 and CDK6 from Cdc37. However, they cooperate with the D-type cyclins to generate CDK4/6-containing ternary complexes that are resistant to cyclin D displacement by Cdc37, suggesting a molecular mechanism to explain the assembly factor activity ascribed to CIP/KIP family members. Overall, our data reveal multiple mechanisms whereby the Hsp90 system may control formation of CDK4- and CDK6-cyclin complexes under different cellular conditions
Collaborator: Laurence Pearl, University of Sussex
Associated publication:
Differential Regulation of G1 CDK Complexes by the Hsp90-Cdc37 Chaperone System (2017) Hallett et al., Cell Repts 21: 1386–1398.


