Section Sidebar
Previous Activities
Activities (2019/04/29 16:55)
Brief Description Members from Stimming Group are keeping active in worldwide events!
Members from Stimming Group are active in worldwide events! Here are some recent activities we have participated in. More exciting things are yet to come!
21st Feb., 2019
Prof. Ulrich Stimming hosted the industry session at Faraday Institution’s 12-month review meeting. Speakers from Jaguar Land Rover, BASF and Johnson Matthey shared their latest achievements and ideas about lithium-ion battery technology.

8th Jan., 2019
On behalf of Prof. Stimming, Dr. Qiaochu Tang gave an invited speech titled “Multi-scale Studies on Li- and Na-ion Intercalation Batteries” at ICESI-PPSS (International Coalition for Energy Storage and Innovation and Pacific Power Source Symposium) in Hawaii. He shared the exciting findings obtained by EC-STM (electrochemical scanning tunnelling microscopy) and machine learning in conjunction with EIS (electrochemical impedance spectroscopy). Thanks to our collaborators in the Technical University of Munich and the University of Cambridge!

2nd Nov., 2018
Prof. Ulrich Stimming gave a seminar titled “Carbon, an Important Material in Energy Process” in the University of Science and Technology of China (USTC), as part of the 60th anniversary seminar series. Prof. Stimming shared his insights on carbon and carbon-based molecules from his long career involving fuel cells, redox flow batteries and lithium-ion batteries.

For details, please visit http://www.sdth1h.com/2018/1019/c2403a341980/page.htm
New Publication! (2017/12/08 09:38)
Brief Description Work of our group with international collaborators was just accepted for publication in Inorganic Chemistry. The title: Mixed-Valent Mn16-containing Heteropolyanions: Oxidation State Tuning and Resulting Physicochemical Properties
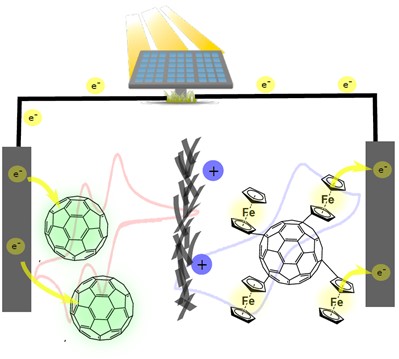
All fullerene-based cells for non-aqueous redox flow batteries
Redox flow batteries have the potential to revolutionize our use of intermittent sustainable energy sources such as solar and wind power by storing the energy in liquid electrolytes. Our concept study utilizes a novel electrolyte system, exploiting derivatised fullerenes as both analyte and catholyte species in a series of battery cells, including a symmetric, single-species system which alleviates the common problem of membrane crossover. The prototype multi-electron system, utilizing molecular-based charge carriers, made from inexpensive, abundant and sustainable materials, principally, C and Fe, demonstrates remarkable current and energy densities and promising long-term cycling stability. The stability of the derivatised fullerenes is assed post-cycling by Matrix-Assisted Laser Desorption/Ionization.
At a conference (2017/06/15 08:50)
Members of the Stimming group will give oral presentations at the International Flow Battery Forum, 27th to 29th of June in Manchester, and the 6th EUROPEAN PEFC & ELECTROLYSER FORUM, 4th to 7th July in Lucerne.
New Publication! (2017/01/20 09:18)
Brief Description Research by the Stimming group and co-workers from Taiwan, Germany and Singapore is published in Phys. Chem. Chem. Phys.
DOI: 10.1039/c6cp05768c
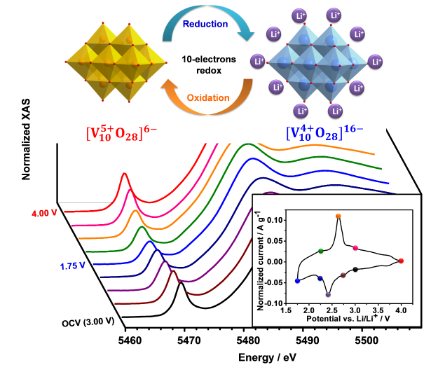
Polyoxometalates (POMs) have been reported as promising electrode materials for energy storage applications due to their ability to undergo fast redox reactions with multiple transferred electrons per polyanion. Here we employ a polyoxovanadate salt, Na6[V10O28], as an electrode material in a lithium-ion containing electrolyte and investigate the electron transfer properties of Na6[V10O28] on long and short timescales. Looking at equilibrated systems, in situ V K-edge X-ray absorption near edge structure (XANES) studies show that all 10 V5+ ions in Na6[V10O28] can be reversibly reduced to V4+ in a potential range of 4–1.75 V vs. Li/Li+. Focusing on the dynamic response of the electrode to potential pulses, the kinetics of Na6[V10O28] electrodes and the dependence of the fundamental electron transfer rate k0 on temperature are investigated. From these measurements we calculate the reorganization energy and compare it with theoretical predictions. The experimentally determined reorganization energy of l = 184 meV is in line with the theoretical estimate and confirms the hypothesis of small values of l for POMs due to electrostatic shielding of the redox center from the solvent.
New Publication! (2017/01/10 13:38)
Brief Description Research by the Stimming group on "Determining Electron Transfer Kinetics at Porous Electrodes" is published in Electrochimica Acta
http://dx.doi.org/10.1016/j.electacta.2017.01.010
-725x241.jpg)
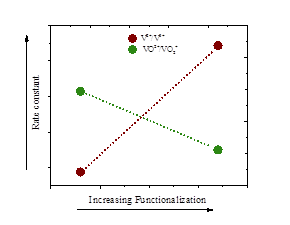
Porous carbon materials are of tremendous importance for electrochemical energy storage. Their low cost, wide potential window and high surface area make them ideal electrodes for many applications. The activity of the electrode towards a certain reaction is given by both the available wetted surface area and the electron transfer constant k0. The present study investigates which electrochemical methods are suitable to determine k0 on porous carbon electrodes. For this purpose, we investigate the ferric/ferrous redox couple on a porous carbon nanotube electrode as model system. We show that results from cyclic voltammetry (CV) can yield an apparent catalytic effect and elucidate its origin. Chronoamperometry and electrochemical impedance spectroscopy are shown to produce consistent values for the exchange current density I0, which can then be normalized to k0. Limitations of both methods in terms of k0 and diffusion constants are discussed. The gathered insights in terms of validity of methods on porous electrodes are harnessed to review the recent literature on the vanadium redox reactions. Reported k0 values spread over four orders of magnitude and there is no consensus on the influence of heat- or acid-treatment on the kinetics. Taking into account the difficulties of CVs on porous electrodes we conclude that reasonable values for the vanadium reactions are k0 < 1:2104 cm s-1 and that oxidation of the samples increases surface area, catalyzes the V2+/V3+ redox reaction but impedes the VO2+/VO2+ redox reaction.
New Funding (2016/12/19 14:52)
Brief Description Group secures research project with Siemens AG
Professor Ulrich Stimming and Dr Jochen Friedl have successfully secured a three-year project with Siemens AG – the largest engineering company in Europe - to investigate electrochemical energy storage.
The grant will see the Newcastle team undertake research on the advancement of redox flow batteries - a rechargeable battery that reversibly converts chemical energy directly to electricity.
For more information, please read the news item on the School of Chemistry Homepage: http://www.ncl.ac.uk/chemistry/about/news/item/chemistrysecureresearchprojectwithsiemensplc.html
New Publication! (2016/10/21 11:20)
Brief Description Researchers from the Stimming Group, Imperial College London, HUST China and TUM CREATE Singapore investigate the double layer structure in Ionic Liquids
http://onlinelibrary.wiley.com/doi/10.1002/celc.201600557/abstract
The presence of water in room temperature ionic liquids (RTILs) can have a strong effect on their properties. In particular, water adsorption at electrodes can reduce the electrochemical potential window of the system. It is thus important to understand where water will be present depending on the electrode potential, the type of ionic liquid and the electrode material. We investigate the influence of water on RTILs by measuring the potential dependent double-layer capacitance of various water-RTIL mixtures. The resulting capacitance versus potential curves is reproduced employing mean-field theory calculations. From the parameters used to obtain the best agreement between experimental and theoretical curves some properties of the RTILs can be deducted, such as a stronger interaction of water with RTIL anions than cations and an agglomeration of water at potentials positive or negative of the potential of zero charge.
New Funding (2016/08/22 20:49)
Brief Description Dr. Friedl was awarded a stipend to attend the Researcher Links workshop in Xi'an China.
Dr. Friedl was awarded a stipend to attend the Researcher Links workshop in Xi'an China. The workshop was sponsored by the British Council and the National Science Foundation of China as part of the Newton Fund and was held from the 15th-18th July 2016. Researchers from the UK and China participated and presented their research to the topics Energy and Environment.

New Publication! (2016/03/18 15:03)
Brief Description New research by the Stimming Group presents new insights in the kinetics of the Vanadium Flow Battery. The question which half-cell is the rate-limiting one is answered unambiguously.
The present study is the first showing that reaction rates for the halfcells are of the same order of magnitude with their respective rate constants depending on the composition of the electrode material. The surface functional groups hydroxyl, carbonyl, and carboxyl on carbon increase the wetted surface area, catalyze the V2+/V3+ redox reaction, but impede the VO2+/VO2+ redox reaction. Commercial graphite felts electrodes were employed for this study.
New PhD Positions (2015/12/09 12:28)
Brief Description Our group has advertised two new PhD positions for a duration of three years. For details please look at the Open Positions sections.
New Funding (2015/11/17 17:11)
Brief Description Prof. Stimming and Dr. Friedl were awarded funding from the Global Excellence Fund to investigate Polyoxometalates with Lasers in Singapore.
Together with Prof. Michel-Beyerle at the Nanyang Technological University, Singapore, our group will employ Femtosecond Absorption Spectroscopy at Polyoxometalates to elucidate the lifetime of excited states in the metal oxide clusters.


New Publication! (2015/09/08 17:00)
Brief Description Well to Wheel Analysis of Low Carbon Alternatives for Road Traffic was accepted

In their study on "Well to Wheel Analysis of Low Carbon Alternatives for Road Traffic"
authors Srikkanth Ramachandran and Ulrich Stimming consider several alternative fuel-vehicle
combinations to replace the internal combustion engine. The manuscript was accepted by
Energy & Environmental Science and is available online, DOI: 10.1039/C5EE01512J
New Homepage! (2015/09/08 14:40)
Brief Description The Stimming Group has a new homepage!
Last modified: Mon, 13 Jan 2020 14:00:44 GMT