Energy
Redox Flow Batteries
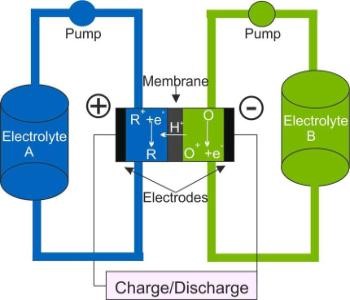
Figure 1. Schematic of a Redox Flow Battery
Redox flow batteries (RFBs) are electrochemical energy storage devices that store energy solely in the electrolyte. They offer many advantages for stationary energy storage. For example, energy and power content can be scaled independently because the former correlates with the size of the tanks, the latter with the surface area of electrodes and membrane. Research in our group includes the investigation of the charge transfer reactions of the most mature RFB, the All-Vanadium RFB.1,2,3
We are also looking at new concepts for RFBs. Polyoxometalates (POMs) are anionic metal-oxide clusters of early transition metals, commonly vanadium, molybdenum and tungsten. We have investigated the application of POMs as active species in RFBs. We demonstrated a POM RFB that uses two POMs, [PV14O42]9- in the positive electrolyte and [SiW12O40]4- in the negative electrolyte.4 These POMs exhibited multi-electron transfers and relatively fast charge transfer kinetics and did not cross cation exchange membranes, which eliminates crossover of active species between half-cells as a source of self-discharge.4
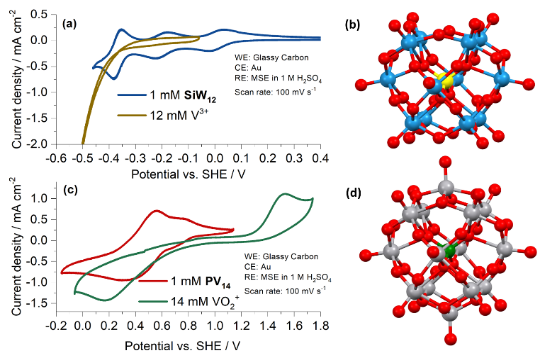
Figure 2. Cyclic voltammograms of the active species of the POM RFB (and of single vanadium ions V3+ and VO2+, the anolyte and catholyte species of the All-Vanadium RFB).4 (a) Comparison of 1 mM [SiW12O40]4- and 12 mM V3+ in 1 M H2SO4 (b) Ball-and-stick representation of [SiW12O40]4- (c) Comparison of 1 mM [PV14O42]9- (1 M LiCl, pH 2.3) and 14 mM VO2+ (1 M H2SO4) (d) Ball-and-stick representation of [PV14O42]9-. (Colour code: silicon = yellow, phosphorus = green, tungsten = blue, vanadium = silver, oxygen = red.)
1. J. Friedl, J, C. M. Bauer, A. Rinaldi, U. Stimming, “Electron Transfer Kinetics of the VO2+/VO2+ Reaction on Multi-Walled Carbon Nanotubes”, Carbon, 2013, 63, 228–239;
2. J. Friedl, U. Stimming, “Determining Electron Transfer Kinetics at Porous Electrodes”, Electrochimica Acta, 2017, 227, 235–245.
3. M. V. Holland-Cunz, J. Friedl, U.Stimming, “Anion effects on the redox kinetics of positive electrolyte of the all-vanadium redox flow battery”, Journal of Electroanalytical Chemistry, 2018, 819, 306–311.
4. J. Friedl, M. V. Holland-Cunz, F. Cording, F. L. Pfanschilling, C. Wills, W. McFarlane, B. Schricker, R. Fleck, H. Wolfschmidt, U. Stimming, “Asymmetric polyoxometalate electrolytes for advanced redox flow batteries”, Energy Environ. Sci., 2018, 11, 3010–3018.
Lithium Ion and Sodium Ion Batteries
Lithium ion batteries (LIBs) are currently the technology of choice for portable electronic devices and battery electric vehicles. We are testing new electrode materials to enhance energy-density and capacity retention during cycling. One concept is that of the molecular cluster battery based on POMs.
Sodium ion batteries are considered as an alternative to LIBs due to the abundance of sodium in the earth's crust, however finding suitable anode materials is still a challenge5,6.
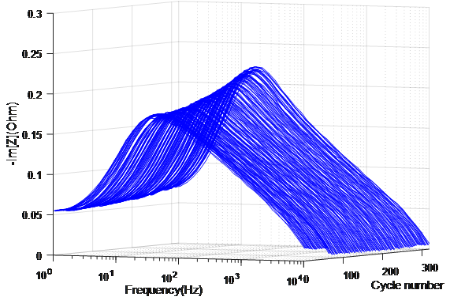
Fig. 3 The evolution of Im[Z] measured on cells at 100%SoC in 25°C as cycle number increases
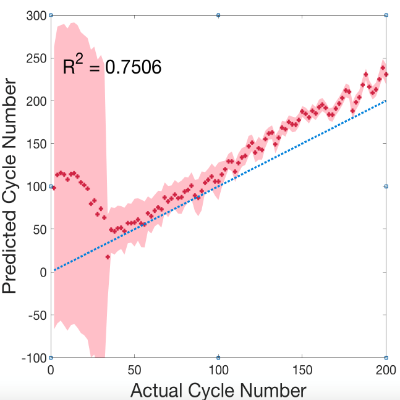
Fig. 4 The identification of cycle number using the universal model
Part of our research on lithium ion batteries (LIBs) is under Faraday Institution’s Degradation Project. In Stimming Group, we focus on the mechanisms of electrochemical degradation and how to find their fingerprints in electrical measurements.
The possibility of diagnosing Li-ion battery’s state of health (SoH) and remaining useful life from electrochemical impedance spectra (EIS) using Machine Learning is investigated in collaboration with Alpha Lee Group in the University of Cambridge7. The Gaussian Process (GP) with automatic relevance determination is used to identify degradation patterns without relying on any equivalent circuit models. The experiment consists of continuous charge-discharge cycling of commercial Li-ion coin cells. With the GP model trained with the entire spectrum measured on identical cells cycled under the same condition, the cycle number of a testing cell can be predicted by providing the corresponding EIS data. Moreover, the trained GP model selected one frequency that shows the most relevant feature to the battery degradation, indicating the potential that battery SoH can be diagnosed by measuring impedance at certain frequency component(s) instead of taking a full-range EIS. With an improved universal model trained with EIS data measured at various SoC under various cycling conditions, the cycle number can be identified without the SoC and temperature information, making the approach promising for real-world battery management and recycling applications.
5. S.Hartung, N.Bucher, H.-Y.Chen, R.Al-Oweini, S.Sreejith, P.Borah, Z.Yanli, U.Kortz, U.Stimming, H.E.Hoster, M.Srinivasan, "Vanadium-based polyoxometalate as new material for sodium-ion battery anodes"; J. Power Sources, 2015, 288, 270–277.
6. H.-Y, Chen, J. Friedl, C.-J Pan, A. Haider, R. Al-Oweini, Y. L. Cheah, M.-H Lin, U. Kortz, B.-J Hwang, M. Srinivasan, U. Stimming, “In situ X-ray absorption near edge structure studies and charge transfer kinetics of Na6[V10O28] electrodes”, Phys. Chem. Chem. Phys., 2017, 19, 3358–3365.
7. Q. Tang, Y. Zhang, A. Lee, U. Stimming, “Machine learning for the diagnosis of battery state of health from impedance spectra”, Oxford Battery Modelling Symposium, 19-20 Mar. 2019, Oxford, UK.
System Analysis
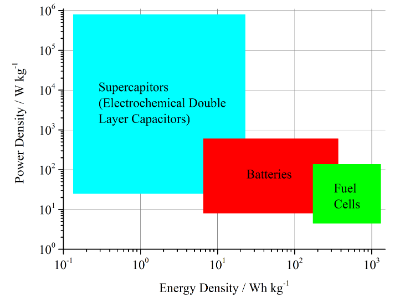
Figure 5. Ragone plot for electrochemical devices.9
High level analysis of energy conversion and energy storage systems with respect to choice of fuels, efficiencies and greenhouse gas emissions. This includes especially the comparison of fuel cells, supercapacitors and batteries for mobile and stationary applications.
One of the main results of our research is that we advocate Bio-ethanol as energy carrier for mobile applications. It can immediately be used in internal combustion engines and later in fuel cell electric vehicles (with and without reformer).
8. S. Ramachandran, U. Stimming, “Well to Wheel Analysis of Low Carbon Alternatives for Road Traffic”, Energy Environ. Sci. 2015, DOI 10.1039/C5EE01512J
9. J. Friedl, U. Stimming, The Importance of Electrochemistry for the Development of Sustainable Mobility. In Energie: Forschung und Konzepte; Bruhns, H., Ed.; Arbeitskreis Energie (AKE) in der Deutschen Physikalischen Gesellschaft, 2014.
Fuel cells
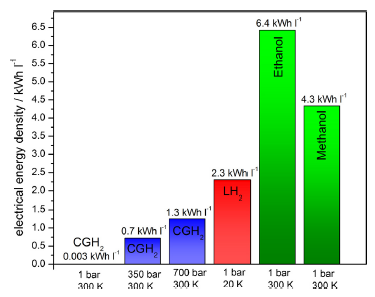
Figure 6. Electrical energy density of several fuels for fuel cells.12
Model electrode studies for the hydrogen evolution reaction (HER), hydrogen oxidation reaction (HOR), ethanol oxidation reaction (EOR) and the oxygen reduction reaction (OER) that are important for fuel cells. Electrochemical studies are combined with spectroscopic tools and microscopy (EC-STM and SECPM). The aim is to obtain fundamental understanding of the mechanisms at the interface and increase the activity of catalysts while decreasing the content of platinum. For this purpose effects of temperature, the support, nano-sized particles and alloying were investigated.
10. W. Ju, M. Favaro, C. Durante, L. Perini, S. Agnoli, O. Schneider, U. Stimming, G. Granozzi, "Pd Nanoparticles deposited on nitrogen-doped HOPG: New Insights into to the Pd-catalyzed Oxygen Reduction Reaction”, Electrochimica Acta, 2014, 141, 89–101.
11. C. Ostermayr, U. Stimming, "Electrocatalytic activity of Pt nanoislands on disordered Au(111) surfaces”, Surface Science, 2014, 631, 229–234.
12. J. Friedl, U. Stimming, “Model catalyst studies on hydrogen and ethanol oxidation for fuel cells", Electrochimica Acta, 2013, 101, 41–58.
13. T. Brülle, O. Schneider, U. Stimming, “Fundamental investigation of the HER on a Pt(111) surface at temperatures down to 120 K", Zeitschrift fuer Physikalische Chemie 2012, 226, 919–934.
Supercapacitors
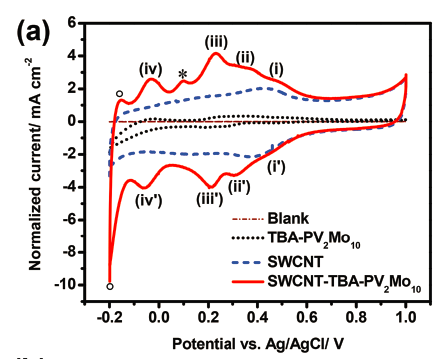
Figure 7. Comparison of single-walled carbon nanotube, polyoxometalate and hybrid electrochemistry
Supercapacitors are electrochemical energy storage devices that can deliver high power. To improve their energy density, we incorporated POMs into the electrodes. POMs add their faradaic pseudo-capacitance to the non-faradaic double layer capacitance of conventional electrochemical capacitors. Because POMs experience multiple electron transfers, facile redox kinetics and are less expensive than traditionally used materials like ruthenium-oxide, they are ideal candidates for supercapacitors.
Together with specialists in POM synthesis we develop systems that improve current devices. One example was a POM anchored on single-walled carbon nanotubes to combat dissolution and therefore capacitance fading.14
14. H.-Y, Chen, R. Al-Oweini, J. Friedl, C. Y. Lee, L. Li, U. Kortz, U, Stimming, M. Srinivasan, “A Novel SWCNT-Polyoxometalate Nanohybrid Material as an Electrode for Electrochemical Supercapacitors”, Nanoscale, 2015, 7(17), 7934–7941.
15. H.-Y, Chen, G. Wee, R. Al-Oweini, J. Friedl, K. S. Tan, Y. Wang, C. L. Wong, U. Kortz, U. Stimming, M. Srinivasan, “A Polyoxovanadate as an Advanced Electrode Material for Supercapacitors”, ChemPhysChem, 2014, 15(10), 2162–2169.