What causes alpha-synuclein aggregation?
To fully understand the role of alpha-synuclein in LBD, a key question is why it accumulates in the first place. In addition to simply accumulating in the brains of individuals with LBD, alpha-synuclein also appears to acquire a relatively unique capacity in becoming "prion-like", meaning it can induce normal alpha-synuclein to similarly stick together, giving the impression it is spreading through biological systems. To elucidate what causes alpha-synuclein aggregation, we adopt a number of approaches:
Alpha-synuclein pathology in neurometabolic diseases
Mitochondrial dysfunction has long been implicated in the pathogenesis of LBD, and many LBD risk genes also cause lysosomal storage disorders, particularly those characterised by sphingolipid accumulation. Therefore, we have been studying alpha-synuclein pathology in neurometabolic diseases in which these processes are implicated. Our previous work has shown Lewy bodies are a relatively common feature of old mitochondrial disease brains, particularly those resulting from nuclear DNA variants, such as POLG1. More recently, we have identified that infants with the sphingolipidosis Krabbe disease manifest "prion-like" alpha-synuclein pathology, constituting the first report of this phenomenon in paediatric brain tissues. We have current studies on-going in metachromatic leukodystrophy and Tay-Sachs disease to further elucidate links between sphingolipids and LBD.
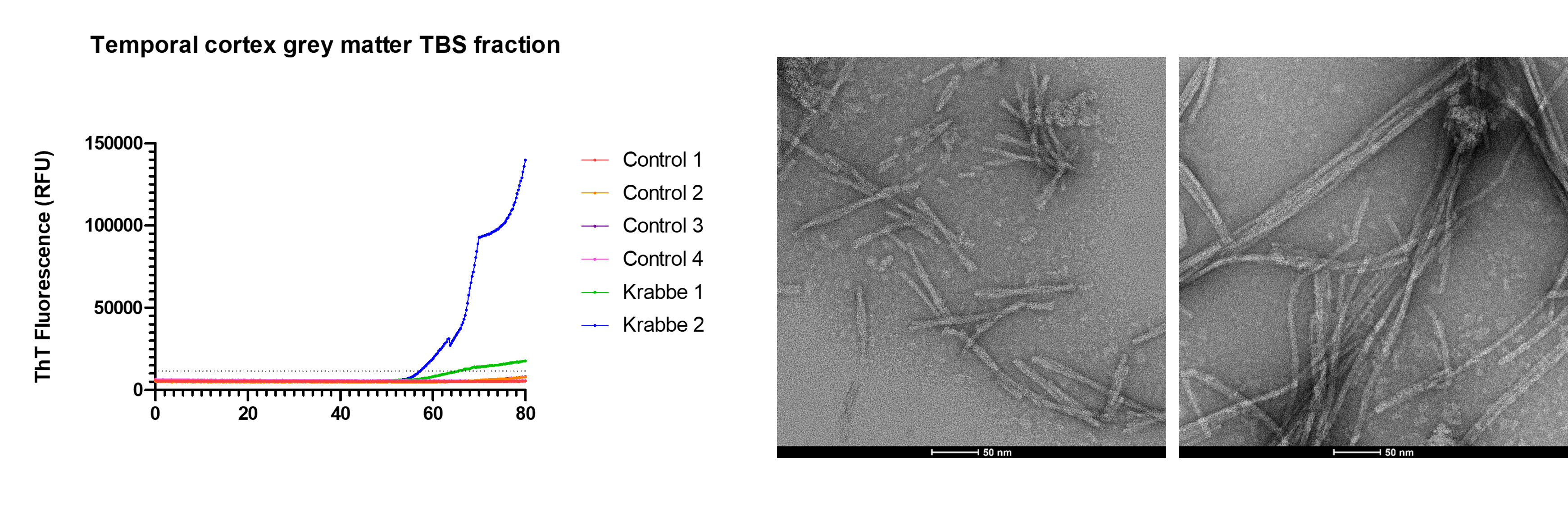
Sphingolipids in LBD
Due to the links we have established between LBD and sphingolipid storage disorders like Krabbe disease, we have started to study sphingolipids in LBD. Combining lipidomics in post-mortem LBD brain tissue with in vitro studies of alpha-synuclein seeded aggregation, Dr Jade Hawksworth aims to determine whether particular sphingolipids associate with risk of alpha-synuclein aggregation.
The developmental trajectory of alpha-synuclein expression
Even in advanced disease, alpha-synuclein aggregation appears to only affect particular populations of neurons whilst others remain remarkably resilient. Understanding what mediates vulnerability to alpha-synuclein is critical to understanding why it occurs and its potential impact on neuronal viability. We have previously identified that brain regions resilient to Lewy body pathology have markedly lower expression of physiological alpha-synuclein, and current studies are exploring the expression of alpha-synuclein over the lifespan.







