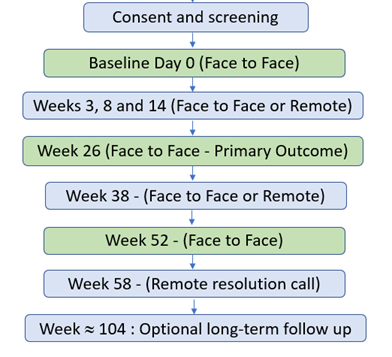
How will the trial be carried out?
Visit 1: Consent, Eligibility and Baseline
Please note, this visit can be split into 2 visits
- Participant consents to take part in the trial
- Participant and trial partner are interviewed by members of the local trial team
- Once participants are confirmed to be eligible, further assessments will be undertaken
- Participant and trial partner to complete questionnaires
- Brief physical exam
- Study drug supplied and participants given a diary to record when they take their study treatment and any adverse events, changes in other medications or visits to the doctors can be recorded
Visit 2: Follow up telephone call (3 weeks)
- Review of information in participant diary with a member of the local trial team over the telephone
Visit 3: Follow up telephone call (8 weeks)
- Review of information in participant diary with a member of the local trial team over the telephone
Visit 4: Follow up telephone call (14 weeks)
- Study drug supplied (delivered or picked up)
- Review of information in participant diary with a member of the local trial team over the telephone
Visit 5: Face to Face Visit (26 weeks)
- Study drug supplied
- Questions about symptoms
- Brief physical examination
- Participant and trial partner to complete questionnaires
Visit 6: Follow up telephone call (38 weeks)
- Study drug supplied (delivered or picked up)
- Review of information in participant diary with a member of the local trial team over the telephone.
Visit 7: Face to Face Visit (52 weeks)
- Questions about symptoms
- Participant and trial partner to complete questionnaires
- Any leftover trial medication collected
Visit 8: End of trial telephone call (56 weeks)
- Side effects or changes since week 52 discussed
- Confirmation of end of the trial
Optional Follow up (24 months)
- Optional follow up for review with a member of the local trial team to review participant medical records or by speaking to the participant and trial partner directly
- Optional consent for the local trial team to have access to participant medical records for up to 10 years after the participant has finished the trial
Flowchart of COBALT Trial Visits