Facilities
General laboratory facilities
We have dedicated tropical and temperate aquaria, available to staff and research students for experiments and for the maintenance of test organisms. The main page for the research aquaria is located on the School of Marine Science and Technology website.
In addition, our group has developed culture facilities for our primary test organism, the barnacle Balanus amphitrite, including a dedicated culture room, a recirculating aquarium system and incubation facilities for the brooding of larvae. Brood stock are provided by the Rittschof lab at Duke, NC. Natural seawater is delivered from the Blue reef Aquarium in Tynmouth and stored in three 1000L tanks where it is constantly circulated, UV and cartridge filtered.
The barnacle 'nauplius' stage is a feeding larva, that requires phytoplankton in order to grow and, ultimately, metamorphose. To ensure a constant and reliable supply of several species of phytoplankton, we have a temperature and light-controlled algal culture room.
Designated algal culture facility: for the production of algae for research. We maintain master cultures of 100ml to 20L carboy batch cultures of single strain algal stocks. Research includes algal biofuels (bio-diesel and bio-ethanol), and chemical signaling. In includes a constant temperature room (19C), with a controlled light:dark cycle of 16:8 hours. Aeration IS provided with air pumps, through Hepa-Vent filters for sterile culture. There is an autoclave, for sterilisation of media and samples and a Microbiological Safety Cabinet, for sterile culture transfer and inoculation. There is also a LED Photobioreactor with 2.5L capacity, for algal culture under controlled environmental parameters.
We are equipped to perform the following molecular techniques; SDS-PAGE gel electrophoresis; 2D-gels; Western and Northern Blotting; PCR and Column Chromatography.
Instruments dedicated to biofouling research
Goniometer
Using probe liquids and a Rame-Hart contact angle goniometer we can determine critical surface tensions for a variety of homogeneous surfaces through contact angle analysis and estimations of polar/non-polar and Lewis acid-base interactions.
Comparison of contact angles taken before and after bioassays allows us to directly observe surface processes that would not otherwise be obvious, for example, the formation of biofilms, reorganisation of surfaces in contact with sea water and determination of material characteristics before and after bioassays.
Large Flow Cell 
The hydrodynamic flow cell was custom built for us by Cussons (Salford, UK) and is used to determine the resistance of attached larvae to hydrodynamic shear. Prof. Martin Downie manages the facility as part of the school’s hydrodynamics laboratory.
Standard glass microscope slides are used as substrata and are held in place by a vacuum during testing. Overall dimensions are 3.3 by 1.8 m with a 15 kW pump delivering up to 90 l/s through the acrylic working section. Maximum bulk velocity is 13.4 m/s (Re = 2.7x105).
The working section measures 2x29x150cm and allows for 6 slides to be measured simulataneously. Wall shear stress developed is proportional to its pressure gradient and, for a 140m ship at 40 knots (Fn = 0.56), this equates to tunnel speed of 13.4 m/s with wall shear stress of 224 Pa.
The system is temperature regulated and controlled via a computer interface.
Push-off machine
Equipment to measure the forces required to remove fouling organisms (usually adult mussels and barnacles) includes a range of hand held force gauges based on a standard method (ASTM5618-2000). In addition, we routinely 'grow on' barnacles to standard sized sample coatings for testing in an automated, computer controlled 'push-off' machine; custom built for our lab by Advanced Analysis and Integration Ltd. (Manchester, UK).
The set up applies a shear force to test adult barnacle attachment strength, however we have recently acquired a custom built hydrodynamic flow cell for determining adhesion strength of cyprids to surfaces.
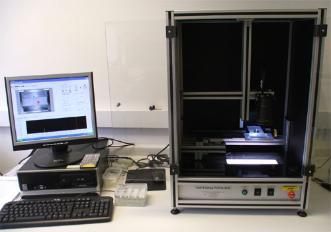
Waterjet
The custom-designed automated water-jetting system is specifically tuned for controlled removal of barnacle cypris larvae (see Aldred et al., 2010). This system generates impact pressures of up to 350 kPa. Areas of interest are designated in advance of each test and for the samples to be jetted in sequence, thus ensuring that all cyprids received equal removal stress.
Spinjet
This equipment is used for the evaluation of novel fouling-release coatings (see Casse et al., 2007), by delivering a jet of water of controlled, variable pressure into the wells of 24-well plates in order to facilitate measurement of the strength of adhesion of algae growing on the base of the wells.
Small flow-cell
Behavioural analysis and tracking equipment
Much of our research concerns the behaviour of marine invertebrates and plants. To monitor behaviours and correlate changes to environmental conditions we have developed a suite of flexible hardware (high-resolution cameras, lenses, infrared illumination) and software packages for 2D (Noldus Ethovision XT9) and 3D (SIMI Reality Motion) tracking. With the advanced capability we have developed, we are thus able to track the movement of objects from the scale of invertebrate larvae (0.5 mm) up to adult fish. For the more challenging applications, such as evaluating the pre-settlement behaviour of barnacle larvae, we also develop specific software packages in-house for both tracking and identification of specific behaviours.
Research microscopy
The laboratories house a number of dedicated research microscopes ranging in power and sophistication from low power stereo ‘scopes for dissection and routine evaluation procedures, through to an advanced inverted fluorescence system. Most of our microscopes are capable of live, high-speed (up to 1000 fps) and time-lapse image capture.
Our principle high-power system is a fully motorised Leica DMi8 inverted microscope. This system has two cameras; one high-resolution monochrome camera for fluorescence microscopy using the 4 fluorescence channels (DAPI, FITC, TRITC, Texas Red), differential interference contrast and phase contrast. A colour camera also allows for publication quality images using standard transmitted light. Multi-point, tile scan, time-lapse and z-stacking modules with 3D deconvolution allow for the design of complex long-term experiments with multiple imaging methods, locations and magnifications to be set up without requiring the operator to be present.