Research
The fertilised egg inherits its nuclear DNA from the female and male gametes - the oocyte and sperm, which become haploid during a specialised form of cell division known as meiosis. By contrast, the mitochondrial DNA is inherited only from the oocyte, which contains some hundreds of thousands of copies. Our research encompasses the inheritance of nuclear and mitochondrial DNA.
Regulation of chromosome segregation during mammalian oogenesis
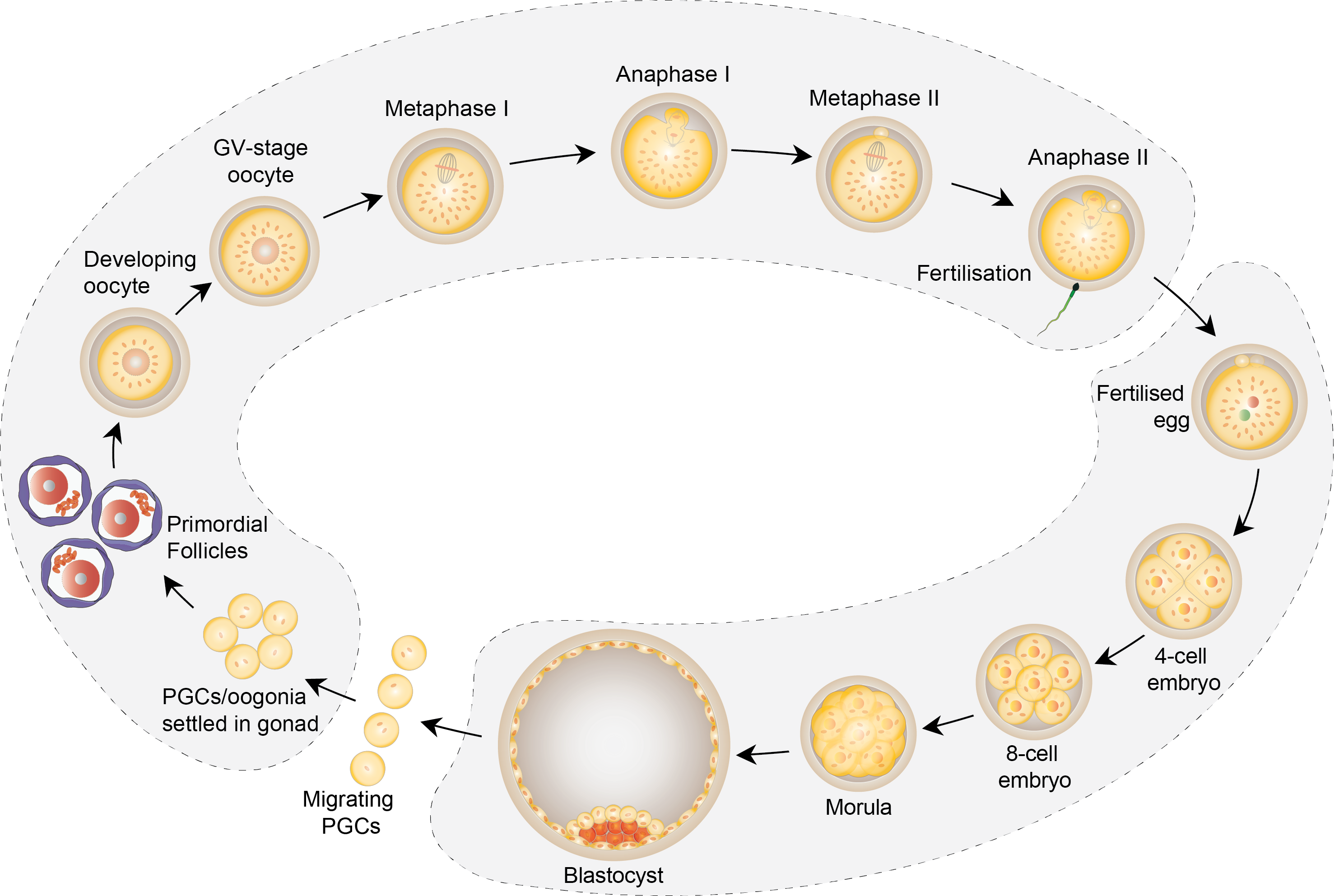
Meiosis generates haploid gametes from diploid germ cells and is essential for sexual reproduction. It involves two rounds of chromosome segregation without an intervening round of DNA replication. The first meiotic division (meiosis I) involves segregation of recombined homologous chromosomes, which have previously established physical linkages (chiasmata) during meiotic recombination to form bivalent chromosomes. Dissolution of cohesion between chromosome arms during anaphase of meiosis I converts bivalents to dyad chromosomes. Dyads consist of two chromatids (at least one recombinant) linked together by cohesion at the centromeres (see Box). Dissolution of centromeric cohesion during anaphase of meiosis II results in segregation of chromatids enabling one copy of each to be inherited by the gamete.
Female meiosis is co-ordinated with the protracted process of oogenesis. Homologous chromosomes recombine in utero but are not resolved until just before ovulation, and meiosis is not finally completed until after fertilisation. The female meiotic divisions are highly asymmetric resulting in the formation of a large oocyte and two tiny, inviable polar bodies. This is a notoriously error-prone process, frequently resulting in too few or too many chromosomes being retained in the oocyte. For reasons that have remained unclear, the risk of error increases greatly as women get older. This results in a dramatic decline in fertility and an increased risk of miscarriage and birth defects from the around the age of 35 years.
We are interested in understanding the molecular links between female ageing and the fidelity of chromosome segregation during the meiotic divisions. We are investigating how the protracted process of female oogenesis affects the unique chromosome structure required for accurate segregation during meiosis (see Box). We use mouse oocytes to elucidate basic mechanisms and we use human oocytes to test the clinical significance of our findings.
This research programme is funded by the MRC.
Development of techniques to prevent transmission of mitochondrial DNA disease.
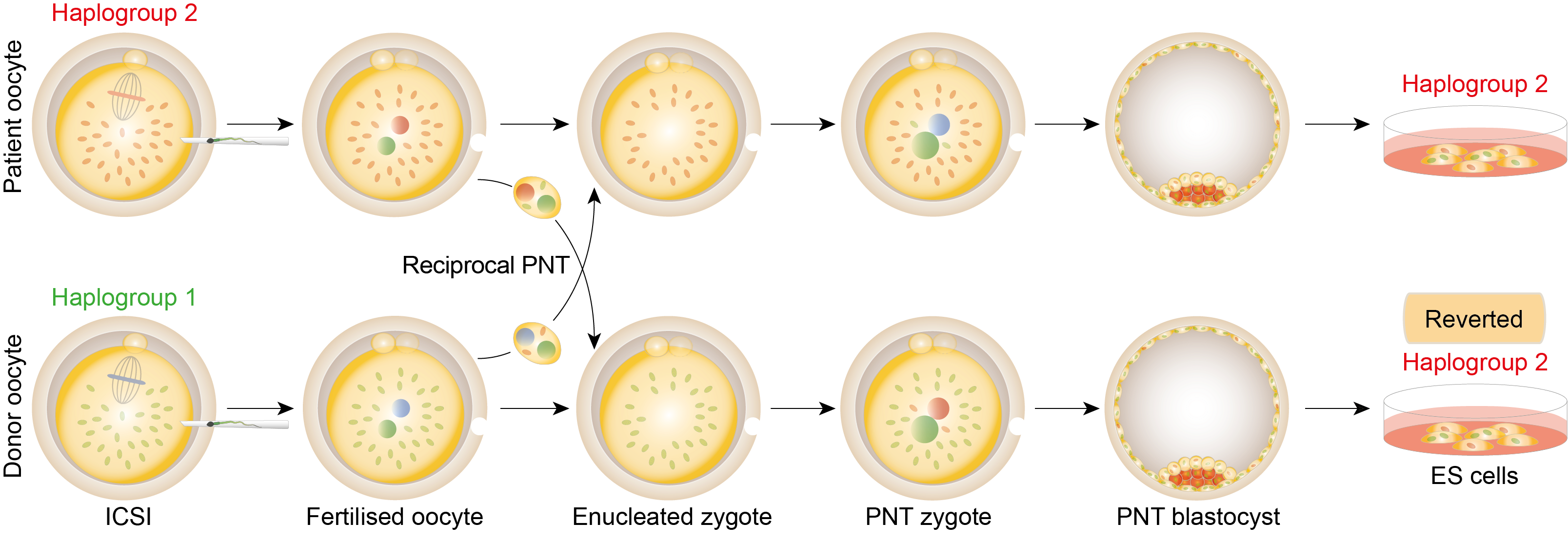
Mutations in mitochondrial DNA (mtDNA) are maternally inherited and can cause a range of serious, debilitating and fatal diseases. This area of research aims to develop techniques to uncouple the inheritance of nuclear DNA and mtDNA, thereby reducing the risk of an affected woman transmitting mutated mtDNA to her child. In the context of clinical treatment, this could be accomplished by transplanting the nuclear DNA from the egg of an affected woman to an enucleated egg from an unaffected donor. In principle, nuclear DNA can be transferred either before (Spindle transfer) of after (Pronuclear transfer).
Proof of concept studies indicate that transplantation of the nuclear genome either before or after fertilisation provides a feasible option for reducing the risk of transmitting mtDNA disease. However, the potential of either approach as future treatments will depend on whether manipulated embryos are capable of undergoing normal development to a stage where they would be capable of implanting in the uterus. Our current research efforts are therefore focussed on optimising procedures to maximise development to the blastocyst stage in vitro, and to perform a range of tests to determine whether those blastocysts are comparable to unmanipulated embryos.
This research programme is funded by the Wellcome Trust.
