Neurosurgical Clinical Trial Unit |
|---|
TOPSAT |
|||||
|---|---|---|---|---|---|
|
|
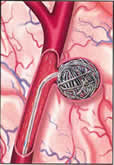
Pilot study comparing early endovascular treatment of aneurysm versus treatment after clinical recovery of patients admitted with WFNS grade IV and V subarachnoid haemorrhage. TOPSAT is a single centre pilot study aimed at comparing early endovascular treatment of aneurysm versus treatment after clinical recovery of patients admitted with WFNS grade IV and V subarachnoid haemorrhage in ITU. 36 patients will be recruited from NGH site over a period of 2 years. | ||||
|
Brief inclusion / exclusion criteria |
|||||
|
Inclusion: Exclusion: |
||||
| Staff Involved |
Principal Investigators: |
Dr.D. Mitra | Trial Co-ordinator: | Mrs. N. Hind | |