Participants
 Sofya Galimova
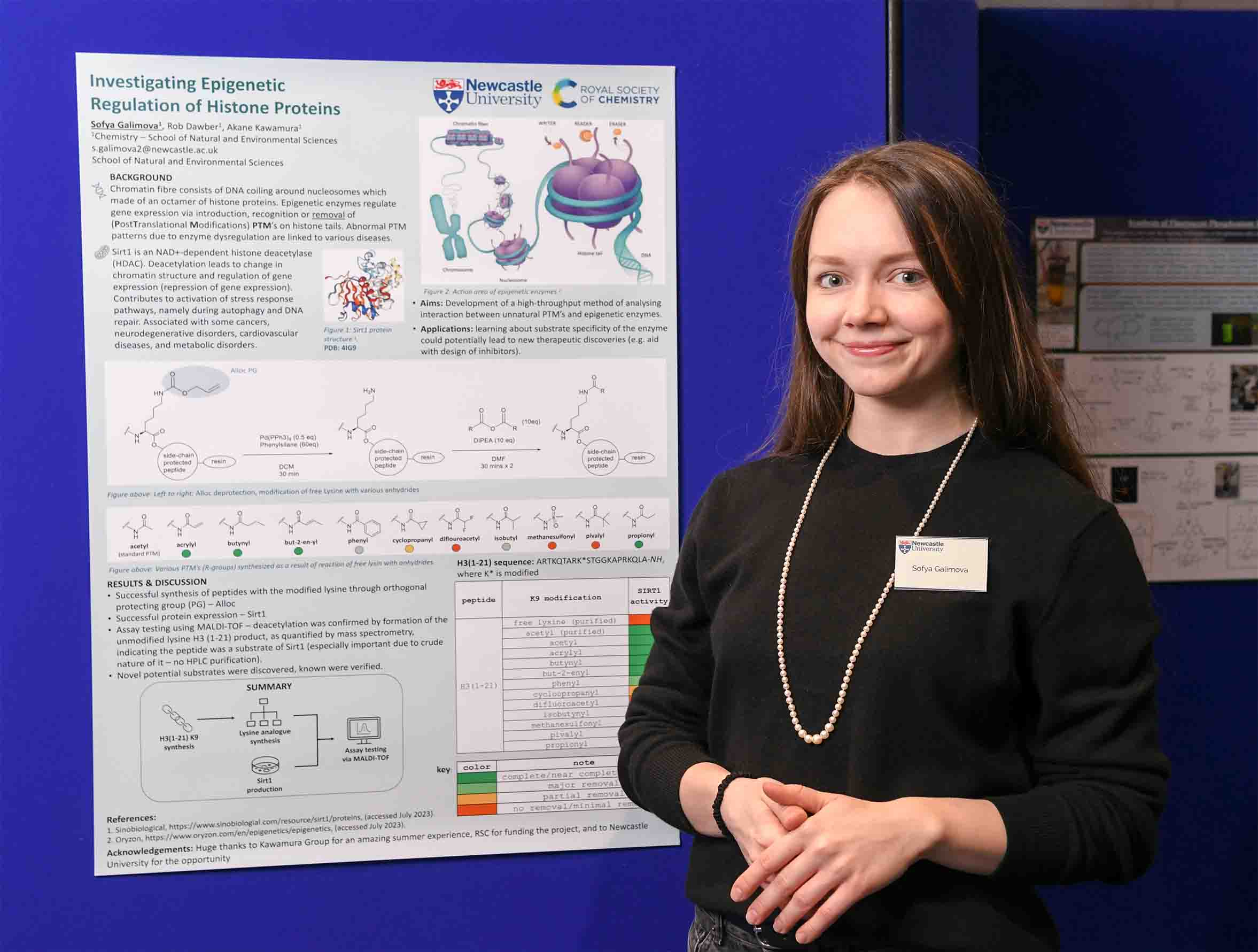
Sofya Galimova
- Investigating Epigenetic Regulation of Histone Proteins
- Mchem Medicinal Chemistry (Industrial Training)
Histones – highly basic proteins found in eukaryotic cell nuclei – prevent DNA from becoming tangled, protect it from DNA damage and play important roles in gene regulation and DNA replication. Post-translational modifications (PTMs) on histone proteins play crucial roles in epigenetic inheritance and regulation of gene expression. Histones are modified and recognised by epigenetic enzymes (known as writers, readers, or erasers) which add, recognize, or remove PTMs. Epigenetic enzymes regulate gene expression via introduction, recognition, or removal of PTMs on histones. Enzyme dysregulation and abnormal PTM patterns are associated with various cancers, thus inhibition of epigenetic enzymes is a basis of both developing and implemented chemotherapies. The aim of this project was to develop a high-throughput method of synthesising a set of atypical lysine modifications on histone H3 peptides, to investigate the substrate scope of Sirt1 (NAD+-dependent histone deacetylase enzyme). After a large-scale synthesis of orthogonally protected H3(1-21) peptide, various lysine analogues were incorporated at Lys-9 using parallel peptide synthesis. Finally, the resulting modifications were examined as substrates of Sirt1 using MALDI-TOF analysis.
Funded by: Royal Society of Chemistry
Project Supervisor: Professor Akane Kawamura
